Allergen Control
Increased awareness of food allergies is leading food companies to develop comprehensive allergen control programs. Here’s what’s involved.
Estimates of the prevalence of food allergies continue to increase. The best current estimates are that 10–12 million consumers in the United States (3.5–4.0% of the overall population) suffer from food allergies (Sicherer et al., 2004). Even as recently as the mid-1990s, the estimate of the percentage of U.S. consumers suffering from food allergies was 1–2%. Consumer perception surveys indicate that an even larger percentage (10% or more) of households believe that one or more members of the household have a food allergy. Regardless of the medical accuracy of these perceptions, these consumers are likely to make purchasing decisions based on these perceptions. So, it’s no wonder that food allergies have become a serious issue for the food industry.
 Is the prevalence of food allergies actually increasing? Although good baseline clinical diagnostic data from earlier decades are missing for comparative purposes, the answer to this question is probably yes. But certainly, the huge jump in the percentage of affected Americans is the result of increased awareness to a greater extent than an actual increase in the prevalence of food allergies. This increase in awareness of food allergies has resulted in increasing assertiveness on the part of food-allergic consumers. Consequently, consumer-response staffs for food companies have seen dramatic increases in the number of telephone inquiries (most are not complaints) from allergic consumers. As expected, consumer product companies are going to respond to this increasing interest in food allergies in part by implementing allergen control strategies.
Is the prevalence of food allergies actually increasing? Although good baseline clinical diagnostic data from earlier decades are missing for comparative purposes, the answer to this question is probably yes. But certainly, the huge jump in the percentage of affected Americans is the result of increased awareness to a greater extent than an actual increase in the prevalence of food allergies. This increase in awareness of food allergies has resulted in increasing assertiveness on the part of food-allergic consumers. Consequently, consumer-response staffs for food companies have seen dramatic increases in the number of telephone inquiries (most are not complaints) from allergic consumers. As expected, consumer product companies are going to respond to this increasing interest in food allergies in part by implementing allergen control strategies.
The situation in the food industry with respect to food allergies continues to be muddled to some degree, however, by the poor understanding of food allergies by food industry professionals and other employees, regulatory officials, and consumers. Many consumers continue to have mistaken beliefs regarding food allergies. In those consumer perception surveys, respondents attributed other food intolerances or even food preferences to food allergies. Food allergies are abnormal responses of the immune system to specific foods that can, at least in some consumers, lead to rather serious manifestations. Other forms of food intolerance, such as lactose intolerance, also occur among many consumers, but these are not true food allergies and do not display such serious symptoms. Thus, a distinction should be made between true food allergies and other forms of food intolerances in food industry allergen management practices, but this is not yet universally happening.
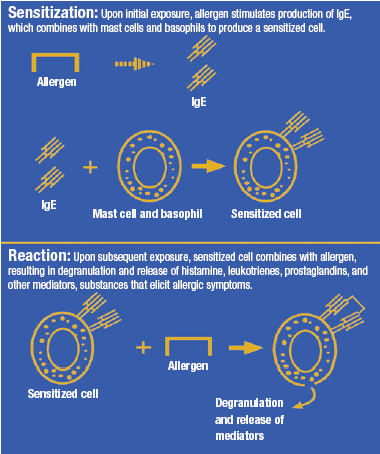
This article will focus on true food allergies, including IgE-mediated reactions and cell-mediated reactions such as celiac disease, but will not cover other types of food intolerance such as lactose intolerance and sulfite-induced asthma (Taylor and Hefle, 2001a).
Designing Allergen Control Programs
The food industry began to implement allergen control programs in the early to mid-1990s. Few, if any, companies had effective allergen control programs in place in 1990, despite the likelihood that food-allergic consumers existed in appreciable numbers even then.
The increasing awareness of food allergies and their potential severity led to the development of allergen control programs, especially over the past 10 years. Many food companies have such programs in place today, although an unknown number of companies have yet to implement these programs. And the effectiveness of these programs is probably quite variable because of their complexity, including such key issues as the diversity of products and ingredients, the range of products made on shared equipment or in shared facilities, the features of processing equipment, including accessibility for allergen cleaning, and the roles of suppliers and custom processors. However, many companies have succeeded in developing highly effective allergen control programs that have lessened the likelihood of allergic reactions among consumers of their products.
The regulatory authorities also began to take notice of food allergies in the early 1990s. Before 1990, very few recalls occurred because of undeclared food allergens. Beginning in the mid-1990s, food allergen recalls became increasingly common. Of course, this regulatory attention also helped to garner the attention of the food industry. However, the number of recalls in recent years is still not an accurate reflection of the existing hazard for food-allergic consumers.Retail surveys conducted by the Food and Drug Administration indicate that about 25% of packaged food products in certain categories contain undeclared residues of peanuts (www.cfsan.fda.gov/~dms/alrgpart.html). If FDA took action on all of these products, the recall numbers could conceivably be much higher. This is also a reflection of the fact that some companies still lack effective allergen control programs.
The development of an allergen control program starts with a risk assessment focusing on some key features of true food allergies:
• True food allergies can cause very serious manifestations. Recent information suggests that 29,000 emergency room visits and 150–200 deaths occur annually from allergic reactions to foods (Bock et al., 2001). While most food-allergic consumers are not at risk of such severe reactions, industry practices should definitely be focused on prevention of severe reactions.
• The most common allergenic foods, sometimes known as “the Big 8,” are milk, eggs, fish, crustacean shellfish (e.g., shrimp), peanuts, tree nuts (almonds, walnuts, etc.), soybeans, and wheat. These eight foods or food groups account for more than 90% of all food-allergic reactions (Taylor and Hefle, 2001a). While hundreds of other foods are less frequently associated with food allergies, the food industry would be wise to focus allergen control programs on the Big 8.
• All of the Big 8 foods except wheat have elicited well-documented fatal reactions. Thus, FDA considers undeclared allergens from the Big 8, with the exception of wheat, as the basis for Class 1 recalls that have the potential for severe health consequences. This regulatory stance is appropriate and serves as another reason for allergen control programs to focus on the Big 8. The U.S. Dept. of Agriculture takes a similar stance with respect to meat and poultry products containing Big 8 allergens.
• Trace amounts of the offending food can elicit allergic reactions in susceptible individuals. While the minimal eliciting doses (also known as threshold doses) are not precisely known, evidence indicates that amounts as low as 1–3 mg of peanut, milk, and egg can elicit mild allergic reactions in the most sensitive individuals (Taylor et al., 2002). While a full discourse on thresholds is beyond the scope of this article, the existence of low provoking doses creates challenging issues in the development of effective allergen control programs.
By addressing the issues appropriately, food manufacturers and suppliers should be able to craft a well-focused approach to allergen management.
Components of a Comprehensive Strategy
A comprehensive allergen control strategy has many components. Quite literally, allergen control is everyone’s job. This task requires total company commitment and recognition of appropriate roles by everyone in the organization. Many companies develop a core team to address the many facets of this issue. However, in smaller companies, individuals often have to play multiple roles in the implementation of a comprehensive allergen control strategy.
• Purchasing. Food manufacturers must know the composition of all raw materials contained in their formulations (Taylor and Hefle, 2000a). While this might sound like a trivial exercise, it is not. The allergen content of all raw materials must be known. Many companies send checklists to suppliers to ascertain if the raw material contains any known allergens, ingredients derived from known allergens, or potential cross-contact allergens from use of shared equipment. This information can yield surprising results; e.g., processors have learned that flavor formulations contain known allergens. Repeated use of these checklists may be helpful to determine if any changes have occurred. Some companies audit suppliers to assess whether compliance with the checklists is satisfactory; this is especially helpful if there is some possibility of cross-contact at the supplier level. The use of allergen test kits to detect residual amounts of allergenic foods can be helpful in the auditing process. Packaging materials should be viewed as raw materials also. Recalls have occurred in the past because of mistakes made on the ingredient statements of packaging materials.
• Receiving. Once allergens have been identified in various incoming materials, the components must be handled appropriately once received by the manufacturing facility (Taylor and Hefle, 2000a). As pointed out by Clark (2005) elsewhere in this issue, dedication and separation are key operations issues in allergen control. However, dedication and separation are also key strategies in receiving. Many companies segregate raw materials containing allergens into distinct warehouse areas—not necessarily separate buildings but separate, divided areas. These areas then can be carefully marked; e.g., color coding together with written product identification can assure that this ingredient will be correctly identified. Allergenic materials should never be stored above non-allergenic materials where a spill might contaminate other ingredients.
Bulk tanks represent a distinct area of concern. If a bulk tank contains an allergenic component, dedication of that bulk tank is an obvious strategy. If that is not possible, then an appropriate sanitation procedure must be developed to clean the tank between allergens and non-allergens.
Care must also be taken with respect to transportation vehicles. Especially with bulk commodities, allergen contamination can occur from previous uses of these vehicles. Our laboratory at the Food Allergy Research & Resource Program (FARRP) once traced peanut contamination of a bakery product to peanut contamination of a rail car that had been used to transport wheat. Control of all such contamination is probably impossible, but gross contamination must obviously be avoided.
• Operations. Manufacturing facilities must often be shared by many products, some containing allergens (Taylor and Hefle, 2000b). Dedication, separation, scheduling, and sanitation are key components of an allergen control plan in such situations.
Of course, dedication of an entire facility or even an entire processing room is only possible in situations where large quantities of a product are manufactured. Some confectionery companies have dedicated certain facilities with respect to peanuts and manufacture no peanut-containing products in those facilities. A few smaller confectionery companies promote their products as peanut-free. This sort of statement requires a very high degree of vigilance. The FARRP Laboratory once traced peanut contamination of a chocolate product to the shared use of jute bags by crop harvesters in the Ivory Coast for cocoa beans and peanuts.
Separation is another widely practiced approach. If entire facilities cannot be dedicated, then perhaps individual processing lines can be. This is especially desirable in processing facilities that are not amenable to wet cleaning, such as bakeries and chocolate manufacturing. For example, cookie manufacturers could have specific baking lines that are devoted either to nonallergenic or allergenic (e.g., peanut or almond) formulations. With adjacent but separate lines, additional precautions are sometimes advisable. Dust transfer is usually not a major concern with the large volumes of material being processed. But if one of the lines is static for a period of time, cleaning may be needed to guard against dust buildup. Heavy plastic separation panels are sometimes used, too.
--- PAGE BREAK ---
If neither dedication nor separation is feasible, then scheduling becomes pivotal. Ideally, allergenic products should be manufactured on shared equipment after non-allergenic products. Allergen cleanup is necessary between manufacturing of allergenic and non-allergenic formulations. Long manufacturing runs are advisable, as this practice limits the number of times that allergen cleanup is needed. Particularly potent allergenic formulations are often manufactured at the end of a shift or a manufacturing cycle, just before major cleanup. Where possible, introduction of the allergenic ingredient into the product later in the process will limit the amount of equipment that must be subjected to allergen cleanup.
Other operations strategies are also important. If in-process totes are used, then some system must be developed to allow their easy identification, such as use of color coding. Furthermore, such totes must be dedicated or effectively cleaned between uses. In some facilities, care must be taken to avoid line crossover that might allow allergen-containing material to fall into other formulations or assure that shielding/covers are in place. Lock-out, tag-out systems have been used effectively to prevent cross-contact in some manufacturing facilities.
The reuse of frying oil for more than one type of product merits some comment relative to allergen control. Shared frying oil is a common practice in the processed foods industry. We are unaware of any allergic reactions attributed to shared frying oil with processed foods, although a few episodes have occurred from similar practices in restaurants (Yunginger et al., 1988). Most companies do not now segregate frying oil, and its use for multiple products is widely practiced. Good manufacturing practices with shared frying oil include use of filtration systems. Although these filters are not designed to remove allergenic proteins, they do remove particulates which would contain allergens. As noted below in the section on sanitation, the potential allergenic hazards associated with reuse of frying oil can perhaps be evaluated by analysis of the next product fried in the oil, using specific immunoassay-based test kits.
• Rework. Several recalls have occurred in the past from misuse of allergen-containing rework in products that were not supposed to contain that particular allergen (Taylor and Hefle, 2000c). We advocate an “exact into exact” approach to use of rework; i.e., rework can only be used in the exact same product from which it was generated. Some companies have developed a policy whereby they either use rework on the day or shift in which it was generated or discard it. Containers for rework must be clearly labeled and easily identified. Many companies use colored totes for allergen-containing rework.
• Sanitation. The sanitation of shared equipment is of paramount importance (Taylor and Hefle, 2000b). Some processing equipment was never designed for easy access and cleaning; e.g., chocolate enrobers or baking ovens. Sanitation advice is particularly hard to provide, given the diversity of products and product contact surfaces in existence in the food industry.
Generally speaking, wet cleaning is preferred where it is feasible. Allergenic proteins tend to be soluble in hot water, although detergents can be useful in removing proteins, too. Cleaning companies have considerable experience with the removal of protein residues and can provide useful advice oriented toward the particular manufacturing facility. Clean-in-place systems are clearly beneficial, where feasible, because they can be made quite consistent once validated.
Dry cleaning systems are much more challenging, but the food industry is replete with examples of situations where wet cleaning is impossible (chocolate confections, baking ovens , etc.). In these situations, wipe-downs are often needed. Air hoses are not especially useful because these devices simply blow allergens from one area to another. Central vacuums are very good because they can effectively remove dry allergen materials without spreading them around.
Push-through is another useful approach in dry cleaning situations. Push-through can be accomplished with the next product or with some “inert” ingredient such as salt or flour (obviously only when undeclared wheat would not be of concern). This approach can be especially useful in places, such as piping systems, where product contact surfaces are completely inaccessible.
--- PAGE BREAK ---
• Sanitation Validation. The validation of sanitation practices on shared equipment is highly recommended. Commercial enzyme-linked immunoassay (ELISA) kits and lateral flow devices (dipsticks) are available from several companies, among them Neogen Corp. (www.neogen.com), r-Biopharm (www.r-biopharm.com), and ELISA Systems (www.elisasystems.net). Test kits can be used to validate sanitation practices.
 Quantitative ELISAs can be done to determine if detectable allergen residues exist in the first product manufactured after changeover, on the assumption that this is the product that is most likely to be contaminated if cleanup is inadequate. However, some companies are reluctant to test finished product on a regular basis. The quantitative ELISAs typically have a lower limit of sensitivity of 1–3 ppm. This level of sensitivity is likely adequate to assure that products are not potentially hazardous to allergic consumers. If the threshold dose is 1 mg of peanut for development of mild allergic reactions in the most sensitive subpopulation and the serving size is 100 g, that would equate to a level of 10 ppm.
Quantitative ELISAs can be done to determine if detectable allergen residues exist in the first product manufactured after changeover, on the assumption that this is the product that is most likely to be contaminated if cleanup is inadequate. However, some companies are reluctant to test finished product on a regular basis. The quantitative ELISAs typically have a lower limit of sensitivity of 1–3 ppm. This level of sensitivity is likely adequate to assure that products are not potentially hazardous to allergic consumers. If the threshold dose is 1 mg of peanut for development of mild allergic reactions in the most sensitive subpopulation and the serving size is 100 g, that would equate to a level of 10 ppm.
Qualitative ELISAs and dipsticks are also available and are typically used to assess whether surfaces are clean. These tests usually have detection limits of 1–5 ppm. Of course, it is impossible to predict how much contamination might exist in the finished product based on a positive swab test of an equipment surface. It is advisable to try to swab areas that are particularly difficult to clean. For many companies, a positive swab test initiates more cleaning until a negative result is obtained.
Some companies have not validated their allergen cleaning practices with test kits. The FARRP Laboratory assisted one company that was concerned about possible allergen cross-contact from use of shared equipment. This company had instituted a very thorough approach to allergen cleanup that required about 6 hr to complete. FARRP was able to verify, using a test kit, that the 6-hr cleanup was effective (no detectable allergen in the first product manufactured after changeover), but was also able to demonstrate that a 45-min cleanup was equally effective. Clearly, the validation exercise was a cost-saving venture for this company.
As noted above, push-through can be an effective strategy in some situations. But validation is particularly important in push-through situations. For example, pushthrough is often used between runs of milk chocolate and dark chocolate. However, in several situations investigated by the FARRP Laboratory, dark chocolate still contained detectable residues of milk, hours after changeover, probably owing to the viscous nature of the material and concentric flow in the piping systems. Accordingly, these manufacturers appropriately decided to use advisory labeling on dark chocolate (e.g., “manufactured on shared equipment with milk”). In this case, only dedicated processing lines can reliably provide milk-free dark chocolate. In other situations, push-through has been effective, although the test kits are the only effective method to determine the volume of push-through needed to prevent allergen cross-contact.
Although some companies have developed in-house laboratory capabilities for testing, many companies rely on contract laboratories. Among others, the nonprofit FARRP Laboratory (www.farrp.org) provides testing services and has access to test methods that are not commercially available, such as those for walnut, pecan, soybean, and others. Most companies can perform the qualitative test methods easily in-house, since they do not require specialized equipment or training.
--- PAGE BREAK ---
• Allergen Auditing. As mentioned previously, auditing has great value in assessing the adequacy of allergen control programs for food manufacturers, including custom processors and ingredient suppliers (Taylor and Hefle, 2000a). Some caution must be exercised, however, because many quality-auditing firms lack allergen training and knowledge. Variability also exists in the quality of the auditing instruments used by various quality auditors. Some companies have trained their own quality auditors, but this option is likely only viable for larger companies. FDA has also conducted some training for its inspectors, although some variability remains between individual inspectors as well.
• Packaging Strategies. Packaging should be viewed as another input that must be controlled as part of the overall allergen control program (Taylor and Hefle, 2001b). Steps must be taken to assure that the product ends up in the intended package and that the ingredient statement on that package is adequate in terms of allergen information. Recalls have resulted from failure to discard old packaging material that subsequently were used for a new formulation containing a new allergenic component.
Precautions should be taken to avoid mixed bundles of packaging. Companies have employed strategies such as using colored striping on the sides of packages so that mixed bundles would be readily evident to the packaging machine operator as he loads the unit.
On large packages of products, the ingredient statements on the outer wrappers should match the ingredient statements on the smaller packages contained inside—allergic consumers often complain of discrepancies in this regard.
• Product Development. Of course, it all starts with product development (Taylor and Hefle, 2001c). The formulation of products containing allergens will lead to the need to adopt allergen control programs in the manufacturing operations. While new products containing allergenic components will likely continue to proliferate, several strategies are worthy of consideration. Ideally, product developers should stay away from the use of allergenic components in cases where other ingredients would work equally well.
Obviously, product developers should avoid use of allergenic ingredients in such minute amounts that they have no functional effect on the finished product. Allergic consumers are often very brand-loyal. So, the reformulation of existing products with the introduction of new allergenic components should be approached with that in mind.
The new Food Allergen Labeling & Consumer Protection Act of 2004 also raises issues regarding ingredients derived from commonly allergenic sources, i.e., the Big 8, as discussed by Trautman (2005) elsewhere in this issue.
Product developers must also be mindful of the allergenicity of ingredients derived from commonly allergenic sources. While labeling of such products is clearly required under the new Act, the need for allergen cleanup must be based on the amount of protein from the allergenic source in the ingredient. For example, if soy lecithin is the only source of soy protein in a formulation, extensive allergen cleanup between the lecithin-containing formulation and another that does not contain any sources of soy protein is probably unnecessary in most cases because the amount of soy protein in lecithin is relatively low; normal cleaning and dilution would resolve allergenic hazards.
--- PAGE BREAK ---
Achieving Effective Allergen Control
The implementation of an effective allergen control program requires considerable effort and total company commitment. While this article has provided some general advice, the elements of effective allergen control vary from one food company to another, based on the nature of the products, the processes used, the ingredients, and the processing equipment.
Perhaps the most important advice is to validate the effectiveness of allergen control approaches using test kits. In some cases, advisory labeling (e.g., “may contain peanuts”) will be required to alert consumers to potential allergenic risks, but FDA has indicated that such labeling cannot be a substitute for Good Manufacturing Practices. Such labeling should be considered as a last resort when other allergen control measures simply are not reliable and when extreme measures like dedicated facilities or equipment are not an economically viable option.
Many companies will need assistance in the development of allergen control programs. FARRP conducts food allergen conferences on a twice-yearly basis (see www.farrp.org for information). These conferences provide the background necessary to begin development of allergen control programs and allow companies to benchmark themselves against industry leaders in allergen control. Assistance with allergen analysis is also readily available from several sources.
Examples of allergy information statements on retail packages
• Contains wheat, milk, soy and peanut ingredients.
• Corn used in this product contains traces of soybeans.
• May contain soy flour, whey, peanuts and tree nuts.
• May contain peanuts or trace amounts of allergens not listed in the ingredients.
• This product may have come in contact with peanuts or other nuts.
• Allergy information: Manufactured on equipment that processes products containing peanuts and other nuts.
• This product contains wheat, milk, eggs and soy. It is made on equipment that also makes products containing tree nuts.
• Manufactured in a facility that processes peanuts.
• Processed on equipment also used to produce products that contain milk, manufactured in a facility that processes nut products.
This work was supported by the Food Allergy Research and Resource Program (FARRP) of the University of Nebraska. A contribution of the University of Nebraska Cooperative Extension Program, Lincoln, NE 68583, Extension Journal Series No. 1027.
Author Taylor is Professor and Co-Director, and author Hefle is Associate Professor and Co-Director, Food Allergy Research & Resource Program, University of Nebraska, Lincoln, NE 68583-0919. Both are Professional Members and Fellows of IFT. Send reprint requests to author Taylor, [email protected].
References
Bock, S.A., Munoz-Furlong, A., and Sampson, H.A. 2001. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 107: 191-193.
Clark, J.P. 2005. Allergen-safe processing. Food Technol. 59(2): 63-64.
Sicherer, S.H., Munoz-Furlong, A., and Sampson, H.A. 2004. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 114: 159-165.
Taylor, S.L. and Hefle, S.L. 2000a. Good manufacturing practices for the food industry—Suppliers and co-packers. Food Allergy Intolerance 1: 208-213.
Taylor, S.L. and Hefle, S.L. 2000b. Good manufacturing practices for allergenic foods—Use of shared equipment. Food Allergy Intolerance 1: 47-50.
Taylor, S.L. and Hefle, S.L. 2000c. Good manufacturing practices for the food industry—Use of rework. Food Allergy Intolerance 1: 114-117.
Taylor, S.L. and Hefle, S.L. 2001a. Food allergies and sensitivities. Food Technol. 55(9): 68-83.
Taylor, S.L. and Hefle, S.L. 2001b. Good manufacturing practices for allergenic foods—Packaging and labeling strategies. Food Allergy Intolerance 2: 53-58.
Taylor, S.L. and Hefle, S.L. 2001c. Good manufacturing practices for allergenic foods—Product development strategies. Food Allergy Intolerance 2: 230-236.
Taylor, S.L., Hefle, S.L., Bindslev-Jensen, C., Bock, S.A., Burks, A.W., Christie, L., Hill, D.J., Host, A., Hourihane, J.O., Lack, G., Metcalfe, D. D., Moneret-Vautrin, D.A., Vadas, P.A., Rance, F., Skrypec, D.J., Trautman, T.A., Yman, I.M., and Zeiger, R.S. 2002. Factors affecting the determination of threshold doses for allergenic foods: How much is too much? J. Allergy Clin. Immunol. 109: 24-30.
Trautman, T. 2005. Labeling food allergens. Food Technol. 59(2): 92.
Yunginger, J.W., Sweeney, K.G., Sturner, W.G., Giannandrea, L.A., Tiegland, J.D., Bray, M., Benson, P.A., York, J.A., Biedrzycki, L., Squillace, D.L., and Helm, R.M. 1988. Fatal food-induced anaphylaxis. J. Am. Med. Assn. 260: 1450-1452.
