Poring Over Membrane Processing Applications
PROCESSING
I recently heard Munir Cheryan, Research Professor in Food Science at the University of Illinois at Urbana-Champaign ([email protected]), present a 45-minute version of his two-day course on membrane processing. This column is a condensation of that abridgement.
Membranes have found applications in many areas of the food industry, including waste treatment, concentration of milk and cheese whey, clarification of beverages, and purification of water. The membranes themselves are made from many different materials with different properties, purposes, and durability. Further, there are many choices in process configuration and membrane module construction.
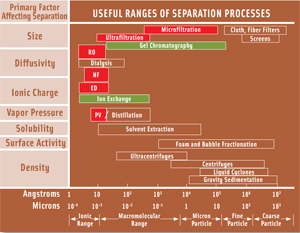
In general, a membrane separation process relies on some barrier, which may be a polymer sheet or tube, or an inorganic material, such as stainless steel or ceramic, to selectively retain some components of a mixture while permitting others to pass through the pores in the barriers. The barrier is characterized by the molecular weight or physical size of the components that are retained. Figure 1 shows the range of retained components and the corresponding process description.
Types of Processes
The tightest membranes are used in reverse osmosis (RO), where ions and small molecules are retained, permitting production of sterile potable water from sea water and brackish streams. Such membranes have been made from cellulose acetate and other polymers. Pressures can exceed 600 psi, and flow rates, conventionally expressed as liters per square meter per hour (LMH), are about 15–25.
At the other extreme are ultrafiltration (UF) or microfiltration (MF), with molecular weight cutoff (MWCO) starting at about 18,000 and reaching pore sizes of 0.45 microns. Nanofiltration (NF) falls between these extremes. Selectivity of membranes is made more complex by the ability of some polymers to dissolve organic molecules that might normally be excluded on the basis of size, but which can diffuse through the membrane. In some cases, molecules that are expected to permeate are adsorbed by the membrane and appear to be rejected, until the adsorption capacity of the membrane is exceeded, and the molecules suddenly break through.
Electrodialysis (ED) and pervaporation (PV) are membrane separations driven by other than pressure forces—ionic charge in the case of ED and vapor pressure in the case of PV. This column focuses on pressure-driven processes.
The transport processes within membranes are complex, involving a combination of hydraulic transport through pores, which often follow tortuous paths, and diffusion through pores and the solid phase of some membranes. In addition, it is normal for a layer of rejected material to build up on the high-pressure side, and this layer, created by concentration polarization, contributes its own resistance to flow and may reduce selectivity of the membrane.
--- PAGE BREAK ---
Design of membrane modules often is intended to promote turbulent flow through thin channels to reduce concentration polarization and increase mass transfer rates. Mass transfer is also affected by viscosity, which tends to increase as the concentration of soluble solids increases. Pressure drop through a channel increases with viscosity and decreases as the dimension gets larger. Pressure drop through the membrane increases with the osmotic pressure difference, increases as viscosity increases, and increases as pore size reduces. Thus, there are compromises influencing the design of modules.
The least-expensive module is spiral wound—in which flat sheets of membrane separated by a spacer are wound around a collection tube. The channel is thin, and a large area of membrane can occupy a relatively small volume. The challenge for a spiral-wound module is suspended solids, which can plug membrane pores and flow channels. Another high-density module is one made from hollow fibers, which may have dimensions close to those of human hair. Pressure can be applied to the outside, or shell side, or to the passage at the center of the fiber. In either case, suspended solids are a problem.
Larger-diameter tubes or plate-and-frame configurations are less sensitive to suspended solids, but occupy larger volumes for the same membrane area. Productivity of membrane processes is related to membrane area, as are the capital costs of the equipment. Operating costs are related to flow rate and pressure, which are achieved with pumps. There are also costs associated with membrane replacement and cleaning. Polymer membranes may need annual replacement, while ceramic or metal membranes, which cost more initially, also last much longer.
The most common process configuration is simple batch with recycle of retentate (the stream of rejected material). Recirculation creates a higher velocity parallel to the membrane surface and thus a higher mass transfer rate. Some retentate is returned to the feed tank. When the material in the feed tank reaches either a target concentration (if the retentate is the product) or such a concentration that the process cannot continue, because the flux rate is too low and the pressure too high, then the process stops, the membranes are cleaned, and a new batch is prepared.
Other configurations are possible to more closely approximate continuous operation. For example, suspended solids can be removed by UF before concentration of the clarified juice by RO. Complex mixtures can be fractionated by selective separation using appropriate membranes and subsequent treatment with other membranes.
Fruit juices, beer, and wine are traditionally clarified by fining or filtering with diatomaceous earth (DE). Fining involves adding gelatin, egg white, or bentonite clay to agglomerate fine particles of cellulose, protein, or phenolics, which make the liquids cloudy. It can take a long time for the haze formers to settle. During that time, spoilage organisms can grow, or if the liquid is cooled to reduce this risk, the rate of settling may also decrease and there is a cost for cooling. Membrane clarification is faster and does not incur the cost of fining agents or DE. In addition, there is a cost to dispose of spent DE, and DE can cause abrasive wear to pumps and seals. Yield of juice is also higher with membrane clarification.
Fruit juices, beer, and wine are traditionally clarified by fining or filtering with diatomaceous earth (DE). Fining involves adding gelatin, egg white, or bentonite clay to agglomerate fine particles of cellulose, protein, or phenolics, which make the liquids cloudy. It can take a long time for the haze formers to settle. During that time, spoilage organisms can grow, or if the liquid is cooled to reduce this risk, the rate of settling may also decrease and there is a cost for cooling. Membrane clarification is faster and does not incur the cost of fining agents or DE. In addition, there is a cost to dispose of spent DE, and DE can cause abrasive wear to pumps and seals. Yield of juice is also higher with membrane clarification.--- PAGE BREAK ---
Some juices are treated with pectinase enzyme to reduce viscosity. Enzyme use can be reduced when clarifying with membranes, though some enzyme is helpful in reducing fouling of the membrane and increasing flux.
Wine, vinegar, and beer are treated with membranes. With wine, the purpose of the membrane treatment is to remove excess tannins; for beer, it is to sterilize the product without heating; and for vinegar, which is made by fermenting wine, it is to remove microbial cells and debris.
Concentration
RO has been used to concentrate the sugars and flavors in juices without the damage caused by heating. There is an upper limit of feasible concentration, set by the pressure tolerance of the membranes and equipment. As the pressure across a membrane increases, the membrane may be compressed, reducing flux.
One interesting approach to reaching higher concentrations involves first ultrafiltering a juice, removing 5–10% of the volume. This stream is pasteurized and later added back to the concentrate. The clear stream is treated with a tight RO membrane, removing 72–76% of the volume as pure water, and giving a concentrate of about 30% solids (starting with 12% solids juice). The concentrate is then processed through “leaky” membranes, producing a permeate with some sugar and flavor and a concentrate of 40–60% solids. The idea is to maintain the same osmotic pressure difference across the membrane. The dilute permeates are returned to the high rejection module.
By my calculations, 42–44% of the feed volume is recycled in a four-stage process that gives a 60% final concentrate. The blended product, with the pulp added, is 43–50% solids and represents 24–28% of feed volume.
As attractive as this process seems, it is apparently not in use. Why might that be? First, it requires membranes resistant to high pressures along with modules containing them that are also built for high pressures. Each module may require a circulation pump and the recycle stream requires a pump handling a significant fraction of the feed volume. The viscosity of high solids concentrates is high, reducing flux and increasing pressure drop through the modules. Nonetheless, the concept is intriguing and has potential for other applications.
Cheryan has developed processes using NF to isolate and concentrate valuable components such as lutein and zeaxanthin from ethanol extracts of corn. The extracts are dilute, and the compounds are 300–1,000 in molecular weight, which makes NF or RO ideal.
The wide range of available membrane materials, the opportunities for process configurations, and the relatively low energy cost of membrane separations mean that there are many unexplored possibilities. With the growing interest in nutraceutical products, there are many potential research topics that involve treating wastes from food processing and replacing thermal concentration processes. Finally, there are many nonaqueous processes, such as vegetable oil and solvent extractions, that are also amenable to membrane applications.
Membranes are not the answer to every situation, but in spite of years of examination, their full potential is still not completely exploited. There is a real opportunity for creative thinking in this area.
by J. Peter Clark
Contributing Editor,
Consultant to the Process Industries, Oak Park, Ill.
[email protected]
