Sensory Testing for Flavorings with Modifying Properties
FEMA Science Committee develops Guidance for the Sensory Testing of Flavorings with Modifying Properties within the FEMA GRASTM Program.

Flavorings with modifying properties (FMPs) are widely used by the flavor industry to modify or enhance the flavor profile of a flavoring and the food to which it is added. In the last few years, the development of new FMPs has increased to help address consumer desire for healthy food alternatives, including reductions in sugar and salt, without compromising flavor. FMPs may not necessarily have or impart a specific characteristic flavor of their own but can modify the flavor profile by altering flavor attributes such as intensifying specific flavor characteristics (e.g., perceived fruitiness), reducing specific flavor characteristics, masking of off-notes or bitterness, or changing the time onset and duration of the perception of specific aspects of the flavor profile.
In the United States, the Expert Panel of the Flavor and Extract Manufacturers Association of the United States (FEMA) evaluates new flavor ingredients, including FMPs, to determine if they can be considered “generally recognized as safe” (GRAS) for their intended use as flavor ingredients under authority provided by the 1958 Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act (Hallagan and Hall, 1995, 2009). The Expert Panel evaluates substances only for their use as flavor ingredients in human food; it does not evaluate substances for other uses in food (e.g., sweetening) or for uses in products other than human food (e.g., tobacco). Therefore, as part of their evaluation, to assure that the flavor ingredient is an appropriate candidate for consideration as FEMA GRAS™, the Expert Panel a) considers if the new flavor ingredient is functioning to impart, enhance, or modify flavor in the finished food product1 under conditions of intended use and b) assesses the effect of the flavor ingredient in the finished food product under conditions of intended use.
To complete their evaluation, the FEMA Expert Panel requires sensory data to be submitted as part of the FEMA GRAS application process for FMPs. In a recent publication in Food Technology (Marnett et al., 2013) the FEMA Expert Panel requested that the flavor industry outline best practices for conducting sensory testing for FMPs to provide data for both items a) and b) above.
FEMA’s Science Committee Sensory Data Task Force, composed of sensory scientists and regulatory experts from FEMA member companies, was formed to respond to the request and developed the document, “Guidance for the Sensory Testing of Flavorings with Modifying Properties within the FEMA GRAS™ Program,” which follows this article.
To provide guidance on whether the FMP functions to impart, enhance, or modify in the finished food product under conditions of intended use [item (a above], the FEMA Sensory Data Task Force developed “Test 1.” Test 1 is used to demonstrate that the FMP does not have inherent sweetness or saltiness under conditions of intended use as an FMP in the finished food product. This test is focused on sweetness and saltiness as the Codex definition1 of flavoring precludes exclusively sweet or salty taste in the finished food product from the definition of flavor2. Additionally, in the United States, if the FMP candidate were exclusively sweet under conditions of its intended use in the finished food, it would not be performing the technical effect of flavor and would require separate regulatory authority to use for that technical effect3.
Test 1 recommends a two-alternative forced choice test (ASTM Designation E2164-08: Standard Test Method for Directional Difference Test) to show that the sweetness or saltiness of the FMP alone and at the maximum use level is less than that of the recognition threshold concentration of sucrose or sodium chloride in the sample matrix evaluated. The guidance provides a recognition threshold concentration of 1.5% for sucrose in a water base, and 0.25% for sodium chloride in a water base4. As these thresholds are only applicable in a water base, the option is provided for the FEMA GRAS applicant to develop a threshold in another food matrix (i.e., meat products) following a three-alternative forced choice standard methodology.
To provide guidance on the assessment of the effect of the FMP on the relevant attributes in the finished food product under conditions of intended use [item b) above], the task force developed “Test 2.” Test 2 recommends a Two-Alternative Forced Choice (2-AFC, also known as Directional Difference Test, Paired Comparison Test), one test conducted for each attribute of interest; scaling methods, such as Descriptive Analysis (e.g., Quantitative Descriptive Analysis, Sensory Spectrum Method); or Time-Intensity Profiling (using standard methodology such as ASTM Designation E2164-08: Standard Test Method for Directional Difference Test; Manual on Descriptive Analysis Testing, R.C. Hootman, Ed., 1992; or ASTM Designation E1909-11: Standard Guide for Time-Intensity Evaluation of Sensory Attributes).
The Sensory Data Task Force is currently evaluating standard food matrices that may be applicable to multiple food categories listed within the FEMA GRAS publications and in the U.S. Code of Federal Regulations (21 CFR 170.3(n)). This work is ongoing and will be available from FEMA when it is completed.
It is anticipated that the “Guidance for the Sensory Testing of Flavorings with Modifying Properties within the FEMA GRAS™ Program” will be a valuable tool for flavor and food companies while providing critical information to the FEMA Expert Panel and others.
--- PAGE BREAK ---
Guidance for the Sensory Testing of Flavorings with Modifying Properties within the FEMA GRASTM Program
Test 1
Inherent Sweetness or Saltiness of FMPs under Conditions of Intended Use
1.1 Objective
This test can be used to demonstrate that the FMP does not have inherent sweetness or saltiness under the conditions of intended use.
1.2 Test Description
Test 1: Is the sweetness or saltiness of the FMP alone (at maximum use level) less than that of the recognition threshold concentration of sucrose or NaCl (or other relevant substance) in the sample matrix evaluated?
- Where the FMP is intended to change a specific attribute(s) or the balance of attributes (e.g., sweetness, saltiness, bitterness, other sensory descriptors, etc.).
- Where the recognition threshold concentration is 1.5% sucrose or 0.25% NaCl (or other relevant substance) in a water base, or the recognition threshold concentration sucrose, NaCl, or other relevant substance in an alternative sample matrix (see section 1.4.2 Recognition Threshold Concentration).
Note: The FEMA GRAS applicant can select alternative relevant substance to sucrose or NaCl or an alternate sample matrix for recognition threshold concentrations; see section 1.4 Method Details, below.
This test may be appropriate if the FMP is intended to modify sweetness, saltiness, or bitterness; or if the FMP is inherently sweet or salty, regardless of whether the FMP is intended to modify sweetness or saltiness. For example, this test would be appropriate to show that a FMP which is intended to mask bitterness is not inherently sweet.
In this test, a Test Sample containing the FMP, which does not contain the ingredient or attribute which it modifies, is compared to a Control Sample which contains the recognition threshold concentration of sucrose or NaCl (or other substance), but which does not contain the FMP. The test(s) should demonstrate that the Test Sample has significantly less sweetness or saltiness than the Control Sample. For further details, see section 1.4 Method Details, below.
1.3 Recommended Method and Standard Methodology
The recommended method is:
- 2-Alternative Forced Choice (2-AFC, also known as Directional Difference Test, Paired Comparison Test)
Standard methodology recommendations include:
- ASTM Designation E2164-08: Standard Test Method for Directional Difference Test
1.4 Method Details
1.4.1 Sample Matrix
The simplest sample matrix is a water base. Additional or alternative relevant sample matrices (simple matrices such as an oil/fat based, alcohol based, or in more complex product matrices) are recommended if the anticipated maximum use level of the FMP in those categories exceeds that determined in water, or if a water base is not relevant.
For example:
- In a water base for a sweet sucrose FMP, a 2-AFC test compares the Test Sample of the FMP alone (i.e., without added sucrose) versus the Control Sample containing 1.5% sucrose.
- In a water base for a salty NaCl FMP, a 2-AFC test compares the Test Sample of the FMP alone (i.e., without added NaCl) versus the Control Sample containing 0.25% NaCl.
If the FEMA GRAS applicant wishes to apply for a maximum use level higher than that determined in a water base, or use a sample matrix other than a water base, then the FEMA GRAS applicant must also determine the recognition threshold concentration of sucrose or NaCl in the chosen matrix, and use that determined threshold concentration for the Control Sample. In the case where the FEMA GRAS applicant chooses to use a sample matrix other than a water base, it is acceptable to use 1.5% sucrose or 0.25% NaCl as the threshold level in the chosen matrix as opposed to determining the threshold concentration of sucrose or NaCl in the chosen matrix. Please see section 1.4.2 Recognition Threshold Concentration, below.
For example:
In a fat based matrix for a sweet sucrose FMP, a 2-AFC test compares the Test Sample of the FMP alone (i.e., without added sucrose) versus the Control Sample containing the recognition threshold of sucrose in a fat based matrix, as determined by the FEMA GRAS applicant.
Please see sections 1.4.3 Control Sample and 1.4.4 Test Sample, below, for further details.
1.4.2 Recognition Threshold Concentration
The recognition threshold concentrations of sucrose and NaCl in a water base have been determined by FEMA to be 1.5% sucrose and 0.25% sodium chloride, respectively.
Should the FEMA GRAS applicant wish to use alternative ingredient(s) to sucrose or NaCl in a water base, or to use an alternative matrix (e.g., simple matrix such as fat/oil based, alcohol based, or a more complex product matrix), the FEMA GRAS applicant may need to make their own determination of the recognition threshold concentration of sucrose, NaCl, or other alternative ingredient(s) relevant to the FMP in question, for each desired alternative sample matrix.
For example:
- A FEMA GRAS applicant who wishes to evaluate a FMP in a water base versus a recognition threshold concentration of aspartame in a water base should determine the recognition threshold of aspartame in that water base.
- A FEMA GRAS applicant who wishes to evaluate a sucrose FMP in a fat based matrix should determine the recognition threshold of sucrose in that fat based matrix.
It is recommended to follow one of the suggested standard methodology documents for determining recognition thresholds. Note: FEMA GRAS applicant is recommended to use 3-AFC methodology to determine recognition threshold within the following standard methodologies:
- ASTM Designation E679: Standard Practice for Determination of Odor and Taste Thresholds By a Forced-Choice Ascending Concentration Series Method of Limits
- ASTM Designation E1432: Standard Practice for Defining and Calculating Individual and Group Sensory Thresholds from Forced-Choice Data Sets of Intermediate Size
- INTERNATIONAL STANDARD ISO 13301: Sensory Analysis Methodology: General guidance for measuring odour, flavour and taste detection thresholds by a three-alternative forced-choice (3-AFC) procedure
Important Note: The recognition threshold determined by the FEMA GRAS applicant may be adjusted by adding one standard error unit to the actual concentration determined. The FEMA GRAS applicant calculates standard error from their study, and uses the determined concentration plus one standard error unit as the concentration of sucrose, NaCl, or alternative ingredient in the Control Sample.
For example:
- A FEMA GRAS applicant determines the recognition threshold concentration of sucrose in a fat-based matrix to be 2.0%. The standard error in the experiment is calculated to be 0.25%. Thus the concentration of sucrose in the fat-based matrix should be 2.0% + 0.25% = 2.25%.
--- PAGE BREAK ---
1.4.3 Control Sample
The Control Sample contains a recognition threshold concentration of sucrose, NaCl, or alternative ingredient(s) without the FMP added. In the cases of using sample matrices other than a water base, or the use of ingredient(s) other than sucrose or NaCl in a water base or other sample matrix, the FEMA GRAS applicant should conduct testing to determine the recognition threshold concentration. In the case where the FEMA GRAS applicant chooses to use a sample matrix other than a water base, it is acceptable to use 1.5% sucrose or 0.25% NaCl as the threshold level in the chosen matrix as opposed to determining the threshold concentration of sucrose or NaCl in the chosen matrix.
For example:
- 1.5% sucrose in a water base without the FMP added.
- 0.25% NaCl in a water base without the FMP added.
- A recognition threshold concentration of an alternative ingredient (plus one standard error unit) in a water base without the FMP added, as determined by the FEMA GRAS applicant.
- A recognition threshold concentration of sucrose (plus one standard error unit) in a sample matrix without the FMP added.
- A recognition threshold concentration of NaCl (plus one standard error unit) in a sample matrix without the FMP added.
1.4.4 Test Sample
The Test Sample contains the FMP alone, without the ingredient it is intended to modify. For example:
- A sweet sucrose FMP alone in a water base without added sucrose.
- A salty NaCl FMP alone in a water base without added NaCl.
- A sweet fructose FMP alone in a sample matrix without added fructose.
- A salty FMP alone in a sample matrix without ingredients that could be modified by the FMP in question.
- A sweet FMP intended to mask bitterness alone in a water base without ingredients that could be modified by the FMP in question.
The concentration of the FMP in the Test Sample should support the conditions of intended use. Note that the use level determined from a sample evaluated in a water base can be applied to all product categories. Should the FEMA GRAS applicant wish to request a maximum use level higher than that determined in a water sample, or wish to test in an alternative sample matrix, they may do so by conducting their testing in alternative sample matrices. Please see section 1.4.1 Sample Matrix, above.
1.4.5 Attribute Tested
The attribute evaluated in the 2-AFC test should be directly related to the intended effect and/or inherent taste quality of the FMP.
- All sweet modifiers should be compared to a sweet Control Sample and tested for sweetness.
- All salt modifiers should be compared to a salty Control Sample and tested for saltiness.
- All bitter modifiers should be compared to a sweet Control Sample and tested for sweetness.
- FMPs not functioning as sweet or salt modifiers (i.e., bitterness maskers), but which are inherently sweet or salty, should be compared to a sweet or salty Control Sample and tested for sweetness or saltiness (respectively).
Consider specifying maximum intensity over a specific period of time if the FMP changes temporal profile of sweetness or saltiness.
Consider the use of nose clips where aroma may interfere with the evaluation of sweetness or saltiness.
1.4.6 Subjects
It is recommended to complete testing with at least 30 responses. The minimum number of subjects is 10, each completing three replicates of the 2-AFC test.
The FEMA GRAS applicant is free to choose naïve, screened, or trained panelists.
Consider screening panelists for anosmia and ageusia.
1.4.7 Data Analysis
The FEMA GRAS applicant is required to demonstrate that the attribute intensity of the Test Sample is significantly less intense than that of the Control Sample.
It is recommended to use the binomial distribution to determine significance in the 2-AFC test with no replicates. Should the FEMA GRAS applicant complete testing with two or more replicates, the FEMA GRAS applicant must use an analysis, such as the beta-binomial, to account for replicates.
The alpha value will be set at 5%. The test should be a two-sided alternative.
1.4.8 Reporting
Reporting of results should include the number of panelists, replicates, frequency of responses, and either calculated p-value (two-sided alternative) demonstrating that p<0.05, or the minimum number of selected responses required for significance at α=0.05 (two-sided alternative), demonstrating the number of responses selecting the Control Sample as more intense exceeds this minimum.
1.5 Sample Test and Results
1.5.1 Example 1
This example demonstrates a 2-AFC test for sucrose sweetness in water.
A FMP intended to modify the sweetness of sucrose was evaluated in a 2-AFC test for sweetness.
Control Sample: 1.5% sucrose in water
Test Sample: 10 ppm FMP in water
Thirty subjects completed a 2-AFC test for sweetness. Twenty-five responses indicated the Control Sample was sweeter. Five responses indicated the Test Sample was sweeter. Using a binomial distribution, the minimum number of responses required for significance at α=0.05 is 21 (two-sided alternative). Therefore, the Control Sample is significantly sweeter than the Test Sample (p<0.05).
This result would suggest a 10 ppm maximum use level in water, which can be applied to any categories desired by the FEMA GRAS applicant.
--- PAGE BREAK ---
1.5.2 Example 2
This example demonstrates a 2-AFC test for sucrose sweetness in an alternative sample matrix.
A FMP intended to modify sweetness of sucrose was evaluated in a 2-AFC test for sweetness.
Control Sample: FEMA GRAS applicant-determined recognition threshold of (in %) sucrose in 5% alcohol base
Test Sample: 25 ppm FMP in 5% alcohol base
Recognition Threshold Determination of sucrose in a 5% alcohol base:
The experiment followed the guidelines of ASTM Standard Method E679-04, for determining recognition threshold of sucrose in a 5% alcohol base. Ten different concentrations of sucrose in a 5% alcohol base were prepared. Each of these samples was presented with two samples of 5% alcohol base. The concentrations were increased by a factor of two per concentration step. Ten panelists completed the test, proceeding from the lower to higher concentrations. At each concentration level, panelists compared the three samples (two blanks and one sucrose sample) and indicate which sample was recognized as being sweet. Each panelist performed the test twice. The best-estimate recognition threshold for sucrose in a 5% alcohol base was found to be AA%1 sucrose.
In a subsequent test, 11 subjects completed three replicates of a 2-AFC test for sweetness. Twenty-four responses indicated the Control Sample was sweeter. Nine responses indicated the Test Sample was sweeter. Using a beta-binomial analysis, p=0.016 (two-sided alternative). Therefore, the Control Sample is significantly sweeter than the Test Sample (p<0.05).
This result would suggest a 25 ppm maximum use level in a 5% alcohol base.
Test 2 Effect of the FMP on Relevant Sensory Attribute(s)
2.1 Objective
This test can be used to demonstrate the intended effect that the FMP has on one or more attributes under the conditions of intended use.
2.2 Test Description
Test 2: Does addition of the FMP cause a significant difference (i.e., increase or decrease) in the sensory attribute(s) being modified?
- Where the FMP is intended to increase or decrease specific attribute(s) or change the balance of attributes (e.g., sweetness, saltiness, bitterness, juiciness, other sensory descriptors, etc.)
- Where attribute is the specific attribute or balance of attributes that is being modified by the FMP (e.g., sweetness, saltiness, bitterness, juiciness, other sensory descriptors, etc.)
In this test, a Test Sample containing the FMP is compared to a Control Sample that does not contain the FMP. The test(s) should demonstrate that the FMP significantly increases or decreases the attribute(s) in question. The attribute(s) chosen and the direction of the difference should support the intended use of the FMP. For example:
- A test using a sweet FMP should demonstrate an increase in sweetness.
- A test using a bitter FMP should demonstrate a decrease in bitterness.
- A test using juiciness FMP should demonstrate an increase in juiciness.
Note that a test using a FMP intended to affect the temporal profile of an ingredient should demonstrate a change (either an increase or decrease) in a temporal attribute(s), but does not necessarily need to demonstrate an increase or decrease in the overall intensity of a relevant attribute. For example:
- A test using a sweet temporal profile FMP can demonstrate a decrease in time to maximum sweetness intensity, but does not need to demonstrate an increase in overall sweetness intensity.
2.3 Recommended Methods and Standard Methodology
The FEMA GRAS applicant can use one or more of a variety of methods to demonstrate significant changes in attribute(s). Each type of method listed could be used to evaluate one or more attributes. Each of the recommended methods has benefits and drawbacks, and the FEMA GRAS applicant is encouraged to employ the method that is best suited to their FMP in question.
The recommended methods are (but are not limited to):
- 2-Alternative Forced Choice (2-AFC; also known as Directional Difference Test, Paired Comparison Test); one test conducted for each attribute of interest; or
- Scaling methods, such as Descriptive Analysis (e.g., Quantitative Descriptive Analysis, Sensory Spectrum Method); or
- Time-Intensity Profiling
Standard methodology recommendations include:
- ASTM Designation E2164-08: Standard Test Method for Directional Difference Test
- Manual on Descriptive Analysis Testing, R.C. Hootman, Ed. 1992
- ASTM Designation E1909-11: Standard Guide for Time-Intensity Evaluation of Sensory Attributes
2.4 Method Details
2.4.1 Sample Matrix
The simplest sample matrix is a water base. Additional or alternative sample matrices (simple matrices such as an oil/fat based, alcohol based, or in more complex product matrices) are recommended to demonstrate efficacy in various product categories, or if a water base is not relevant (Appendix A). The sample matrix should contain the ingredient(s) and/or attribute(s) on which the modifier is effective. Please see section 2.4.2. Control Sample, below, for examples.
2.4.2 Control Sample
The Control Sample contains some level of the ingredient(s) or attribute(s) with which the proposed FMP is effective, but that does not contain the modifier. For example:
- A sample matrix containing some level of sucrose without the sweet sucrose FMP added.
- A sample matrix containing some level of NaCl without the salty NaCl FMP added.
- In the case of a bitter masker or blocker: A sample matrix containing perceptible bitterness without the bitterness FMP added.
- In the case of a juiciness FMP: A sample matrix containing the ingredient(s) to be modified, but without the juiciness FMP added.
The FEMA GRAS applicant may include more than one Control Sample, if desired. For example:
- Additional samples containing differing concentrations of relevant ingredients.
--- PAGE BREAK ---
2.4.3 Test Sample
The Test Sample is the Control Sample to which the FMP has been added. The concentration of the FMP in the Test Sample should support the conditions of intended use.
- A sample matrix containing the same level of sucrose as the Control Sample, with the sweet sucrose FMP added.
- A sample matrix containing the same level of NaCl as the Control Sample, with the salty NaCl FMP added.
- In the case of a bitter masker or blocker: A sample matrix with perceptible bitterness, containing the same ingredients as the Control Sample, with the bitterness FMP added.
- In the case of a juiciness FMP: A sample matrix containing the ingredient(s) to be modified, containing the same ingredients as the Control Sample, with the juiciness FMP added.
The FEMA GRAS applicant may include more than one Test Sample, if desired. For example:
- Additional samples containing differing concentrations of FMP.
2.4.4 Attribute(s) Tested
The attribute(s) evaluated in the test(s) will be directly related to the intended effect of the FMP. For example:
- All tests with sweet modifiers should evaluate sweetness.
- All tests with salt modifiers should evaluate saltiness.
- All tests with bitter modifiers should evaluate bitterness.
- All tests with juiciness modifiers should evaluate juiciness.
Note that if a FMP is intended to affect the temporal profile of an ingredient, the attribute(s) tested should include a temporal attribute(s), but does not necessarily need to demonstrate an increase or decrease in the overall intensity of a relevant attribute. For example:
- A test using a sweet temporal profile FMP can demonstrate a change (either increase or decrease) in time to maximum sweetness intensity.
- A test using a sweet temporal profile FMP can demonstrate a change (either increase or decrease) in duration of sweet intensity.
For additional examples of temporal profile attributes, please see the recommended Time-Intensity testing methodology in section 2.3 Recommended Methods and Standard Methodology, above.
2.4.5 Subjects
2.4.5.1 2-AFC Testing
It is recommended to complete testing with at least 30 responses. The minimum number of subjects is 10, each completing three replicates of the test. The FEMA GRAS applicant is free to choose naïve, screened, or trained panelists.
2.4.5.2 Descriptive Analysis Testing
The FEMA GRAS applicant is referred to standard methodology for appropriate number of subjects and training procedures for panelists; see section 2.3 Recommended Methods and Standard Methodology, above. The FEMA GRAS applicant is free to choose naïve, screened, or trained panelists.
2.4.5.3 Time-Intensity Testing
The FEMA GRAS applicant is referred to standard methodology for appropriate number of subjects and training procedures for panelists; see section 2.3 Recommended Methods and Standard Methodology, above. The FEMA GRAS applicant is free to choose naïve, screened, or trained panelists.
2.4.6 Data Analysis
The FEMA GRAS applicant is required to demonstrate that the intensity of the Test Sample is significantly different than that of the Control Sample for the attribute(s) being modified.
The direction of the difference may depend on the type of flavor modification being sought:
- A test using a sweet FMP intended to increase sweetness should demonstrate that the Test Sample is significantly sweeter than the Control Sample.
- A test using a bitter FMP intended to mask or block bitterness should demonstrate that the Test Sample is significantly less bitter than the Control Sample.
- A test using a sweet temporal FMP intended to decrease the time to maximum sweetness intensity should demonstrate that the time to maximum sweetness intensity of the Test Sample is significantly less than that of the Control Sample.
The alpha value will be set at 5% for determining significant differences.
2.4.6.1 Analysis of 2-AFC Test Data
It is recommended to use the binomial distribution to determine significance in the 2-AFC test with no replicates. Should the FEMA GRAS applicant complete testing with two or more replicates, the FEMA GRAS applicant must use an analysis, such as the beta-binomial, to account for replicates.
--- PAGE BREAK ---
2.4.6.2 Analysis of Descriptive Analysis Test Data
It is recommended to use a t-test for each attribute when evaluating a total of two samples. Analysis of Variance (ANOVA) is recommended for each attribute when evaluating more than two samples. Additional factors may be incorporated in ANOVA calculations (such as panelists, replicates, etc.).
If ANOVA is used for statistical calculations, a multiple comparison test should be employed to specify differences among three or more samples (such as Fisher’s LSD, Tukey’s HSD, etc.).
2.4.6.3 Analysis of Time-Intensity Test Data
It is recommended to use a t-test for each attribute when evaluating a total of two samples. Analysis of Variance (ANOVA) is recommended for each attribute when evaluating more than two samples. Additional factors may be incorporated in ANOVA calculations (such as panelists, replicates, etc.).
If ANOVA is used for statistical calculations, a multiple comparison test should be employed to specify differences among three or more samples (such as Fisher’s LSD, Tukey’s HSD, etc.).
2.4.7 Reporting
2.4.7.1 2-AFC Test
Reporting of results should include the number of panelists, replicates, frequency of responses, and either calculated p-value (two-sided alternative) demonstrating that p<0.05, or the minimum number of selected responses required for significance at α=0.05 (two-sided alternative), demonstrating the number of responses selecting the Control Sample as more intense exceeds this minimum.
2.4.7.2 Descriptive Analysis
Reporting of results should include the number of panelists, replicates, description of methods and attributes evaluated, and a table of mean responses including the lettering convention representing significant differences in attribute(s) (p<0.05) using a multiple comparison test of the FEMA GRAS applicant’s choice. Figure(s) such as histogram(s), spider plot (if measuring multiple attributes), etc. may be included with significant differences in attributes clearly identified.
2.4.7.3 Time-Intensity Test
Reporting of results should include the number of panelists, replicates, description of method used and attribute definitions, and a table of mean responses including the lettering convention representing significant differences in attribute(s) (p<0.05) using a multiple comparison test of the FEMA GRAS applicant’s choice. Figure(s) such as time-intensity curves may be included with significant differences in attributes clearly identified.
2.5 Sample Test and Results
2.5.1 Example 1
This example demonstrates 2-AFC testing and binomial test results.
A FMP intended to modify sweetness of sucrose was evaluated in a 2-AFC test for sweetness.
Control Sample: 5% sucrose in water
Test Sample: 5% sucrose in water containing 10 ppm sweet sucrose FMP
Thirty subjects completed a 2-AFC test for sweetness. Twenty-two responses indicated the Test Sample was sweeter. Eight responses indicated the Control Sample was sweeter. Using a binomial distribution, p=0.016 (two-sided alternative). Therefore, the Test Sample is significantly sweeter than the Control Sample (p<0.05).
2.5.2 Example 2
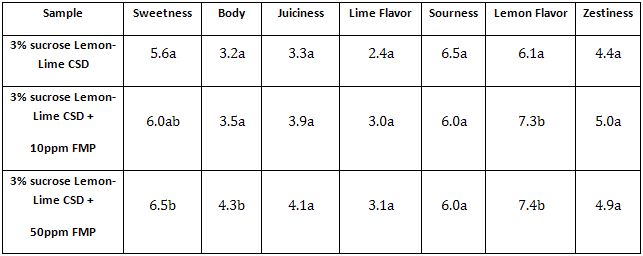
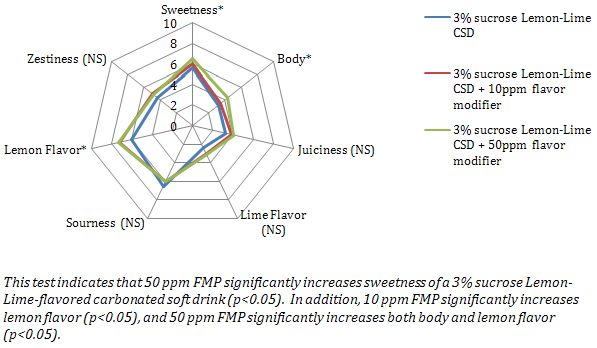
This example demonstrates Descriptive Analysis Testing and ANOVA results.
A FMP intended to modify sweetness of sucrose is evaluated in a Descriptive Analysis test including sweetness and other attributes of interest.
Control Sample: 3% sucrose in a Lemon-Lime flavored carbonated soft drink (CSD)
Test Sample 1: 3% sucrose in a Lemon-Lime flavored carbonated soft drink (CSD) containing 10 ppm FMP
Test Sample 2: 3% sucrose in a Lemon-Lime flavored carbonated soft drink (CSD) containing 50 ppm FMP
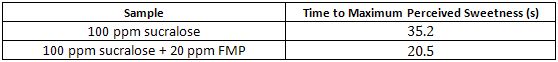
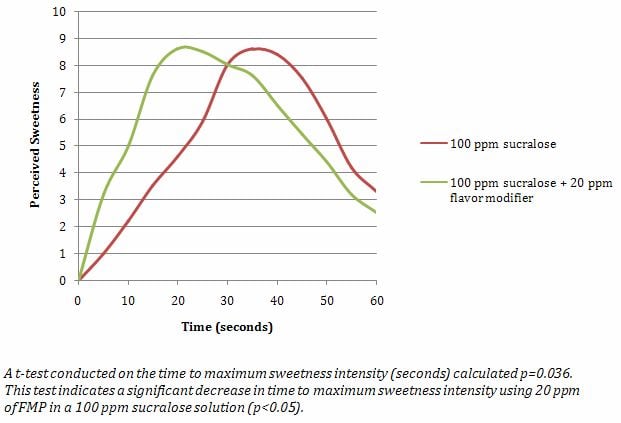
2.5.3 Example 3
This example demonstrates Time-Intensity Testing and t-test results.
A FMP intended to modify the temporal profile of sucralose was evaluated in a Time-Intensity test for sweetness.
Control Sample: 100 ppm sucralose in a water base
Test Sample: 100 ppm sucralose in a water base containing 20 ppm FMP
The experiment followed the guidelines of ASTM Standard Method E1909-11. The panel (n=15) was familiar with conducting time-intensity evaluations. The sensory panel evaluated the samples in three replicate sessions using a 15-point scale for sweetness (0 = none, 15 = strong). The samples were dispensed in 30 ml volumes, labeled with three-digit blinding codes, and served in random order. Panelists evaluated sweetness continuously in the mouth for 60 seconds. Samples were expectorated at 60 seconds. Seltzer water and unsalted crackers were provided for palate-cleansing between the samples. The data was collected by using computerized data collection software. Statistical analyses was conducted on the time to maximum sweetness intensity (in seconds) for each sample using a t-test (p<0.05).
3 Example Carried Through Both Tests
3.1 Example 1
This example demonstrates testing for a FMP which is intended to mask bitterness.
Test 1:
A FMP intended to modify (mask) bitterness was evaluated in a 2-AFC test for sweetness.
Control Sample: 1.5% sucrose in water
Test Sample: 10 ppm FMP in water
Fifteen subjects completed two replicates of a 2-AFC test for sweetness. Twenty-two responses indicated the Control Sample was sweeter. Eight responses indicated the Test Sample was sweeter. Using a beta-binomial analysis, p=0.012 (two-sided alternative). Therefore, the Control Sample is significantly sweeter than the Test Sample (p<0.05).
This result would suggest a 10 ppm maximum use level in water, which can be applied to any categories desired by the FEMA GRAS applicant.
Test 2:
A FMP intended to modify (mask) bitterness was evaluated in a 2-AFC test for bitterness.
Control Sample: 5% aspartame in water
Test Sample: 5% aspartame in water containing 10 ppm FMP
Ten subjects completed three replicates of a 2-AFC test for bitterness. Twenty-one responses indicated the Control Sample was more bitter. Nine responses indicated the Test Sample was more bitter. Using a beta-binomial analysis, p=0.043 (two-sided alternative). Therefore, the Control Sample is significantly more bitter than the Test Sample (p<0.05).
Christie L. Harman, Corresponding Author, is associated with the Flavor and Extract Manufacturers Association, 1620 I Street., NW, Suite 925, Washington, D.C. 20006 ([email protected]). John B. Hallagan is Legal Advisor to the FEMA Expert Panel.
NOTES
1 The Codex Alimentarius Guidelines for the Use of Flavourings (CAC/GL 66-2008) defines flavorings as “products that are added to food to impart, modify, or enhance the flavour of food with the exception of flavour enhancers considered as food additives under the Codex Class Names and the International Numbering System for Food Additives - CAC/GL 36-1989. Flavourings do not include substances that have an exclusively sweet, sour, or salty taste (e.g., sugar, vinegar, and table salt). Flavourings may consist of flavouring substances, natural flavouring complexes, thermal process flavourings, or smoke flavourings and mixtures of them and may contain non-flavouring food ingredients within defined conditions such as carriers, solvents, etc. Flavourings are not intended to be consumed as such.”
2 Sour taste is also included but was not included in this guidance.
3 Technical effect refers to the function of a food ingredient in food. Technical effect F05, flavors and flavor modifiers, refers to substances that impart, supplement, intensify, or modify the taste and/or aroma of a food. This category excludes [technical effect] of sweeteners (National Academy of Sciences, 1989).
4 These recognition thresholds were derived from a literature search of articles citing thresholds for taste sensations related to sweetness and saltiness. The FEMA Sensory Data Task Force filtered the literature by: 1) requiring articles citing “recognition thresholds,” not “detection thresholds,” with the reasoning that the sensation needs to be recognized as sweet or salty and 2) sample size of greater than or equal to 20 subjects/observations.
5 To be determined by FEMA GRAS applicant.
FEMA Science Committee Sensory Data Task Force
David Tonucci, Givaudan, Chair; Fred Shinnick, Senomyx, Vice- Chair; Eyassu Abegaz, Ajinomoto North America; Tess Aldredge, McCormick and Co.; Petra Baker, Symrise; Polly Barrett, Kalsec; John Cavallo, Citrus and Allied Essences; Jason Cohen, Tate & Lyle; Hyung Chang, Chromocell; Denver Christopher, Citrus and Allied Essences; Dolf DeRovira, Flavor Dynamics; Tanya Ditschun, Senomyx; Doug Eisenoffer, Kerry Ingredients and Flavours; Imad Farhat, Firmenich; Rudy Fritsch, Chromocell; Scott Hagedorn, Kerry Ingredients and Flavors; John Hightower, The Coca-Cola Co.; Suzanne Johnson, McCormick and Co.; Deborah Kennison, Symrise; Silvia King, McCormick and Co.; Lore Kolberg, Tate & Lyle; Susanne Paetz, Symrise; Dorothy Panhorst, Firmenich; Uma Parasar, International Flavors and Fragrances; Teresa Pendergast, Takasago; Debby Poskanzer, WILD Flavors; Sidd Purkayastha, PureCircle; Wei Qin, International Flavors and Fragrances; Colin Ringleib, PepsiCo; Miro Smriga, Ajinomoto North America; Srini Subramanian, Firmenich; Jennifer Tartaglia, Takasago; Donna Thede, Kellogg; and Amanda Warnock, Givaudan.
References
Hallagan, J.B. and Hall, R.L. 1995. FEMA GRAS—A GRAS assessment program for flavor ingredients. Regul. Toxicol. Pharmacol. 21: 422.
Hallagan, J.B. and Hall, R.L. 2009. Under the conditions of intended use—new developments in the FEMA GRAS program and the safety assessment of flavor ingredients. Food Chem. Toxicol. 47: 267.
Marnett, L.J., Cohen, S.M., Fukushima, S., Gooderham, N.J., Hecht, S.S., Rietjens, I.M.C.M., Smith, R.L., Adams, T.B., Hallagan, J.B., Harman, C., McGowen, M.M., and Taylor, S.V. 2013. GRAS Flavoring Substances 26: The 26th publication by the Expert Panel of the Flavor and Extract Manufacturers Association provides an update on recent progress in the consideration of flavoring ingredients generally recognized as safe under the Food Additive Amendment. Food Technol. 67(8): 38-56.
NAS. 1989. 1987 Poundage and Technical Effects Update of Substances Added to Food. National Academy of Sciences, Washington, D.C.
