Deconstructing the Methylmercury Myth
U.S. dietary guidelines are clear: the benefits of consuming a variety of seafood outweigh theoretical mercury risks.

Every five years since 1980 the U.S. Dept. of Agriculture convenes the best minds in nutrition and related fields to form the Dietary Guidelines Advisory Committee (DGAC). During 20 months of deliberations, the 2010 DGAC examined several issues along with the following concerns about seafood:
1. Do health outcomes vary depending on the source of omega-3 fatty acids (marine versus plant sources)?
2. What effect does the consumption of fish and fish-derived omega-3 fatty acids have on the risk of cardiovascular disease?
To address these concerns, the 2010 DGAC reviewed 25 articles published between June 2005 and March 2010. The committee evaluated the articles on strength of evidence according to quality (scientific rigor and validity), consistency of findings, quantity of studies and number of subjects in the studies, clinical impact, and generalizability to the population of interest. For example, the committee gave an overall grade of “moderate” to evidence supporting omega-3 fatty acids from plant sources. A plant-sourced alpha-linolenic acid (ALA) intake of 0.6% – 1.2% of total calories meets recommendations and may lower the risk of cardiovascular disease, but newer evidence is insufficient to warrant intake beyond this level. However, limited research suggests that higher intake of omega-3 fatty acids from plant sources may reduce mortality among persons with existing cardiovascular disease.
Other research supports the intake of omega-3 fatty acids through the consumption of seafood. Research by Mozaffarian and Rimm (2006) provides strong evidence that modest consumption of seafood containing 250 mg – 500 mg of eicosapentaenoic acid (EPA) and docosa-hexaenoic acid (DHA) is associated with reduced risk of death from cardiovascular disease. As a consequence, the 2010 DGAC concluded that two servings per week of seafood (three to five ounces per serving) providing an average of 250 mg/day of omega-3 fatty acids from this source would result in the kind of cardio-protective benefits observed by Mozaffarian and Rimm. In support of seafood for maternal and child health, the 2010 DGAC classified as moderate the evidence supporting the consumption of omega-3 fatty acids during pregnancy and lactation: In particular, the committee noted that DHA had been associated with improved infant health outcomes such as visual acuity and cognitive development. Moreover, the work of Hibbeln (2007) demonstrated that a high percentage of children have lower verbal IQs when their mothers had low (approximately 0.05%) omega-3 fatty acid consumption at 32 weeks of pregnancy.
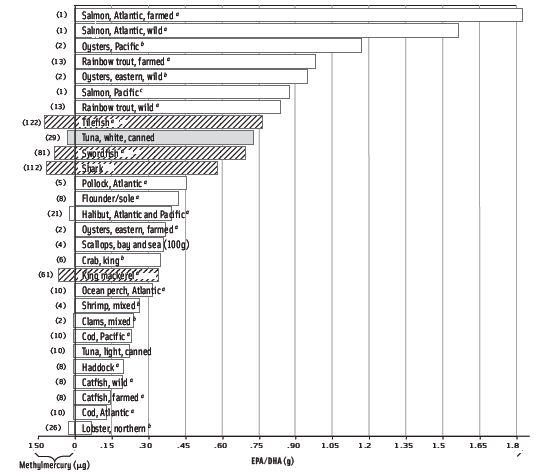
According to the 2010 DGAC, consistent moderate evidence shows that the health benefits from consuming a variety of cooked seafood outweigh hypothetical risks. All consumers—including women who are or may become pregnant, nursing mothers, and children age 12 or younger—can safely eat at least 12 ounces of seafood per week, provided they follow local seafood advisories. However, pregnant and nursing women should avoid four large predatory fish: swordfish, shark, tilefish, and king mackerel. This recommendation is consistent with a report by the Institute of Medicine (2007).
--- PAGE BREAK ---
Seafood’s Tarnished Reputation
Seafood has not always enjoyed such an honored position. Previous DGACs merged seafood with other muscle proteins without a focused review of nutritional benefits unique to the consumption of seafood. In addition, two industrial incidents that resulted in methylmercury poisoning of seafood resulted in the unfair elevation of the risks of seafood consumption over the benefits. Neither incident involved commercial seafood in the United States: The first incident occurred in the 1930s when Chisso Corporation began dumping mercury into Minamata Bay off the coast of Japan (Harada, 1995). The mercury moved into the food chain and bioaccumulated from phytoplankton (algae) to small fish to large predatory fish. Fish and shellfish in Minamata Bay were a primary source of protein for Minamata’s residents. Cases of methylmercury poisoning began surfacing in the 1950s with thousands of victims experiencing brain damage, loss of sight and/or hearing, numbness, tremors, and other problems. The second incident occurred in 1971 when methylmercury-based fungicide in grain supplied to Iraq for planting was instead consumed because the planting season had ended (Bakir et al., 1973).
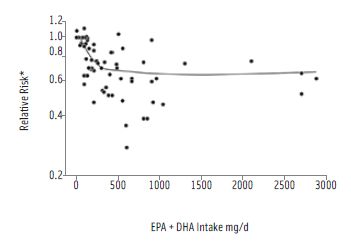 While the levels of mercury present in Japan and Iraq were on a scale dramatically different than the levels seen in commercial seafood in the United States (the range of methylmercury in U.S. seafood is .01 ppm – 1.4 ppm), the devastating effects of the poisonings, specifically on developing babies, piqued the scientific community’s interest in studying societies that eat seafood-rich diets (IOM, 2007). In 2000 the U.S. National Research Council reviewed three prospective epidemiological studies on methylmercury exposure and neurodevelopment in children in the Faroe Islands, Seychelles Islands, and New Zealand. Maternal hair levels of methylmercury were measured in each study and compared to a variety of behavioral outcomes. A positive correlation between fish consumption and methylmercury exposure was reported for the Faroe Islands and New Zealand studies but not for the larger Seychelles study. The Seychellois children showed no consistent pattern of adverse associations present between prenatal methylmercury exposure and neuro-cognitive and behavioral testing. Despite prenatal methylmercury exposure (due to maternal intake of large quantities of seafood), there was evidence of improved performance on some neuro-cognitive and behavioral endpoint tests in the range of total mercury exposure studied (0.54 ppm – 23 ppm), a finding that reflects the role of beneficial nutrients present in fish as demonstrated in previous studies (Davidson et al., 2011). The Seychellois population consumed primarily small reef species of fish.
While the levels of mercury present in Japan and Iraq were on a scale dramatically different than the levels seen in commercial seafood in the United States (the range of methylmercury in U.S. seafood is .01 ppm – 1.4 ppm), the devastating effects of the poisonings, specifically on developing babies, piqued the scientific community’s interest in studying societies that eat seafood-rich diets (IOM, 2007). In 2000 the U.S. National Research Council reviewed three prospective epidemiological studies on methylmercury exposure and neurodevelopment in children in the Faroe Islands, Seychelles Islands, and New Zealand. Maternal hair levels of methylmercury were measured in each study and compared to a variety of behavioral outcomes. A positive correlation between fish consumption and methylmercury exposure was reported for the Faroe Islands and New Zealand studies but not for the larger Seychelles study. The Seychellois children showed no consistent pattern of adverse associations present between prenatal methylmercury exposure and neuro-cognitive and behavioral testing. Despite prenatal methylmercury exposure (due to maternal intake of large quantities of seafood), there was evidence of improved performance on some neuro-cognitive and behavioral endpoint tests in the range of total mercury exposure studied (0.54 ppm – 23 ppm), a finding that reflects the role of beneficial nutrients present in fish as demonstrated in previous studies (Davidson et al., 2011). The Seychellois population consumed primarily small reef species of fish.
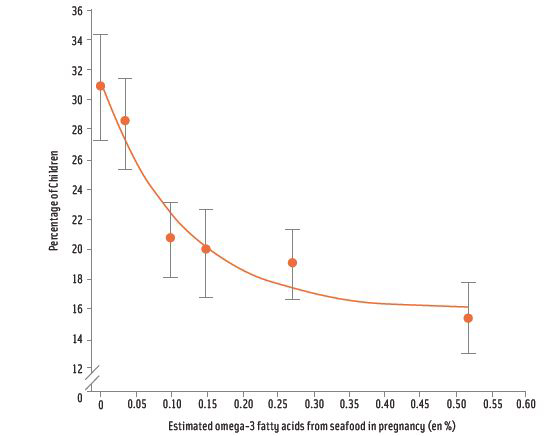 In the study looking at maternal seafood intake of women in the Faroe Islands in the North Atlantic, two out of 10 IQ-related tests had negative neurological results (Rice, 2000). However, residents of the Faroe Islands consumed primarily the meat of pilot whales, which is not common in the American diet. Moreover, a whale is not a fish. Subsequent adjustment of the Faroe Islands data to isolate the effects of seafood from whale meat also showed no adverse effects from eating fish. Thus, use of the original Faroe Islands study in setting dietary guidelines for seafood was not appropriate. As a comparative average, U.S. women of child-bearing age are exposed to methylmercury levels far less than women in the Seychelles Island study and those in the Faroe Islands study; the same is true for U.S. children’s exposure to methylmercury (McDowell et al., 2004).
In the study looking at maternal seafood intake of women in the Faroe Islands in the North Atlantic, two out of 10 IQ-related tests had negative neurological results (Rice, 2000). However, residents of the Faroe Islands consumed primarily the meat of pilot whales, which is not common in the American diet. Moreover, a whale is not a fish. Subsequent adjustment of the Faroe Islands data to isolate the effects of seafood from whale meat also showed no adverse effects from eating fish. Thus, use of the original Faroe Islands study in setting dietary guidelines for seafood was not appropriate. As a comparative average, U.S. women of child-bearing age are exposed to methylmercury levels far less than women in the Seychelles Island study and those in the Faroe Islands study; the same is true for U.S. children’s exposure to methylmercury (McDowell et al., 2004).
The 2010 DGAC’s approach to examining the net benefit of seafood consumption is now becoming common in the published literature. Contemporary fish consumption advisories must balance the risks and benefits instead of only considering risk. As Stern and Korn (2011) note, the risk of methylmercury exposure and the benefits of omega-3 fatty acids are not accurately quantifiable without taking each into account. However, communicating benefits versus risks to consumers and health professionals is a challenge.
 Framing the Message
Framing the Message
Scientifically proven benefits are often clouded with enough myths about risks—particularly through social media—that consumers pause before making purchasing decisions. Communicators agree that the messaging should be science-based, clear, and consistent. Seafood is a nutrient-dense protein and the best dietary source of omega-3 fatty acids EPA and DHA. ALA from plants is another dietary source of omega-3 fatty acids and is often added to foods such as orange juice and margarine. Recent estimates of how much ALA is converted to EPA and DHA range from less than 1% to about 5% (Williams and Burdge, 2006). In addition, evidence by Conquer and Holub (1997) suggests a limited retro-conversion of DHA to EPA among vegetarians and omnivores. From public health and policy development perspectives, research has unequivocally demonstrated the positive relationship between seafood and coronary health in adults and cognitive and visual development in infants and children. Although consumers who eat raw or under-cooked seafood risk exposure to pathogens, those who do not consume seafood or restrict their consumption to a low intake experience lifelong health risks.
--- PAGE BREAK ---
A group of seafood professionals identified the 2004 FDA-EPA advisory “What You Need to Know about Mercury in Fish and Shellfish” as a key contributor to the confusion. Surveys taken in 2009 showed that most registered dietitians, nurses, and doctors recognized the health benefits, but more than 50% of health professionals interpreted the advisory’s message as negative (Hicks et al., 2013). Seafood consumption by mothers plummeted because doctors and nurses did not understand the advisory. The website “Seafood Health Facts” (www.seafoodhealthfacts. org), which was primarily the result of two focus groups held at Oregon State University’s Food Innovation Center, helped change prevailing attitudes toward seafood. Designed for consumers as well as health care professionals, the website takes a tiered approach to customize seafood consumption information by providing advice on the best way to meet U.S. dietary guidelines while consuming seafood as a source of protein. Additionally, the website provides information on seafood and health, seafood sustainability, seafood choices, and market issues.
The 2010 DGAC deconstructed the mercury myth of seafood and urged all consumers to eat at least eight ounces a week for cardio-protective benefits, improved health outcomes for infants and children, and better quality of life.
R. Clemens, a Professional Member of IFT, is Adjunct Professor, USC School of Pharmacy, Los Angeles, Calif. 90089, and Chief Scientific Officer, Horn, LaMirada , Calif. 90638 ([email protected]).
A.W. Hayes, a member of IFT, is Visiting Scientist, School of Public Health, Harvard Univ., Cambridge, Mass. 02115 ([email protected]).
D. Hicks, a member of IFT, is Seafood Technology Specialist, Univ. of Delaware, Lewes, Del. 19958 ([email protected]).
M. Morrissey, a Professional Member of IFT, is Director, Food Innovation Center, Oregon State Univ., Portland, Ore. 97209 ([email protected]).
B. Blakistone, a Professional Member of IFT, is Sr. Director, Scientific Affairs, National Fisheries Institute, McLean, Va. 22102 ([email protected]).
References
Bakir, F., Damluji, S.F., Amin-Zaki, L., Murtadha, M., et al. 1973. Methylmercury poisoning in Iraq. Science 181(4096): 230–241.
Conquer, J.A. and Holub, B.J. 1997. Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores. Lipids 32(3): 341–345.
Davidson, P.W., Cory-Slechta, D.A., Thurston, S.W., Huang, L.S., et al. 2011. Fish consumption and prenatal methylmercury exposure: cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology 32(6): 711–717.
Harada, M. 1995. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 25(1): 1-24.
Hibbeln, J.R., Davis, J.M., Steer, C., Emmett, P., et al. 2007. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369(9561): 578–585.
Hicks, D.T., Pivarnik, L.F., Richard, N.L., Gable, R.K., and Morrissey, M.T. 2013. Assessing knowledge and attitudes of U.S. healthcare providers about benefits and risks of consuming seafood. J. Food Sci. Educ.12 (4): 75–80.
IOM. 2007. Seafood Choices: Balancing Benefits and Risks. Institute of Medicine. National Academies Press, Washington, D.C.
McDowell, M.A., Dillon, C.F., Osterloh, J., Bolger, P.M., et al. 2004. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ. Health Persp. 112 (11): 1165–1171.
Mozaffarian, D. and Rimm, E.B. 2006. Fish intake, contaminants, and human health: evaluating the risks and benefits. J. Amer. Med. Assoc. 296(15): 1885–1899.
NRC. 2000. Toxicological effects of methylmercury. National Research Council. National Academies Press, Washington, D.C.
Rice, D.C. 2000. Identification of functional domains affected by developmental exposure to methylmercury: Faroe Islands and related studies. Neurotoxicology 21(6): 1039–1044.
Stern, A.H. and Korn, L.R. 2011. An approach for quantitatively balancing methylmercury risk and omega-3 benefit in fish consumption advisories. Environ. Health Persp. 119(8): 1043–1046.
Williams, C.M. and Burdge, G. 2006. Long-chain n-3 PUFA: plant v. marine sources. Proc. Nutr. Soc. 65(1): 42–50.
