Reducing Added Sugars with Polyols
Polyols or sugar alcohols deliver multiple functionalities in products, including sweetness, bulk, calorie reduction, solubility, and cooling effect.

Americans are in a constant struggle to maintain and/or reduce their weight. At last count, according to the Centers for Disease Control and Prevention (CDC), one-third of U.S. adults and 17% of youth are considered obese (Body Mass Index >30), which has stabilized in recent years but is still very alarming. One of the key recommendations in the fight against obesity, according to the U.S. Dept. of Agriculture’s (USDA) 2015–2020 Dietary Guidelines for Americans, is to reduce the amount of added sugars consumed from products such as snacks and sweets. Surprisingly, consumers are aware of this fact, but find it easier said than done. Choosing sugar-free or reduced-sugar substitutes for traditional, calorically dense comfort foods may help individuals achieve long-term weight loss success.
Recent changes in U.S. food labeling regulations have increased emphasis on reduction of added sugars and calories. Traditional food products—such as sweet baked goods or confections—can be reformulated as no-sugar-added, reduced sugar, and sugar-free. For the product developer, however, just supplying any functional product will not do the trick. To satisfy the consumer, reduced-sugar products need to be similar in appearance, taste, and quality to their full-sugar counterparts. And although creating a similar product can be a challenge, it’s not the only challenge to consider. The developer must also design a product that can be manufactured with relative ease—in most cases using existing processing equipment—as well as one that is cost effective to produce.
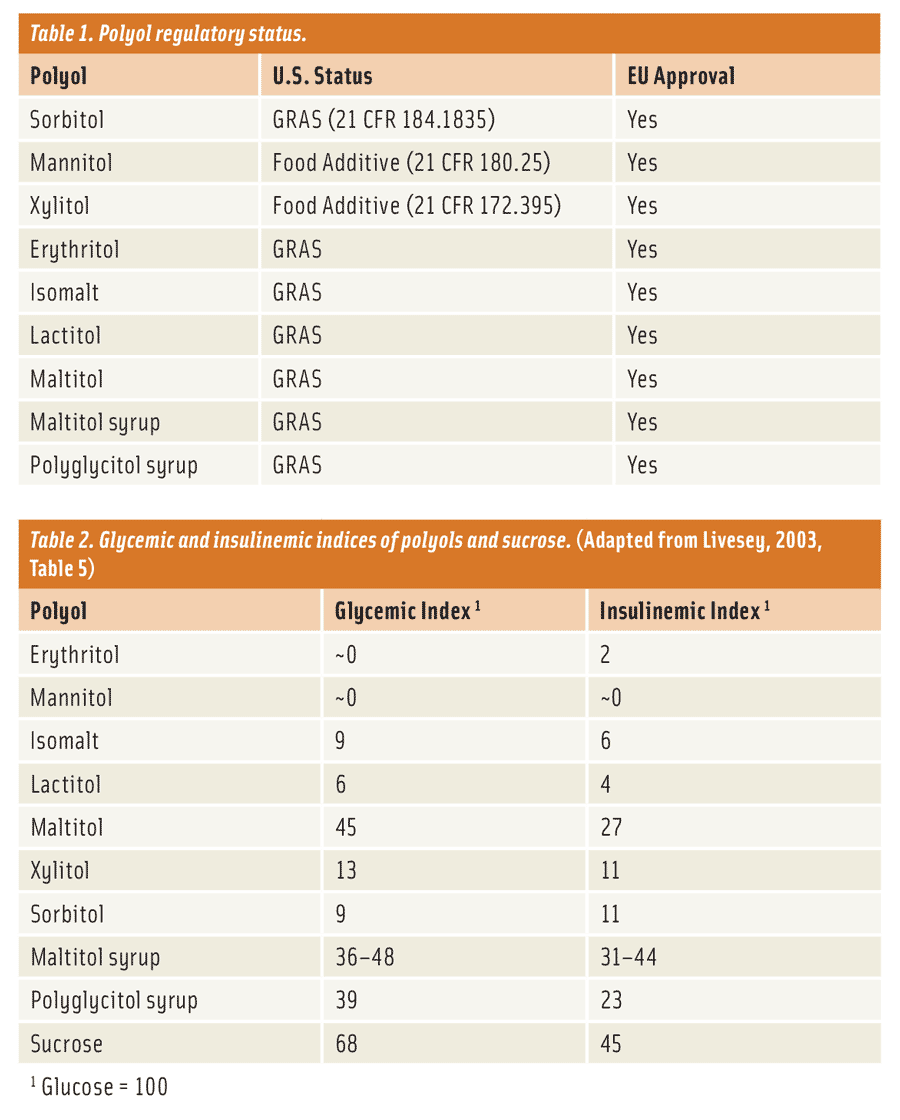
What does a product developer use to create these no-sugar-added, reduced-sugar, and sugar-free products? The most obvious answer is to use something that offers the same bulk and functionality as sugar. This might seem like a difficult task, but it’s not. There is a unique group of low-digestible carbohydrates—not considered sugars (21 Code of Federal Regulations [CFR] 101.9)—called polyols or “sugar alcohols” (Table 1). They are ideal sugar replacements, providing bulk and adding functionality to just such applications, with a number of physiological benefits. Apart from their dental properties (polyols are non-cariogenic), one of the main benefits of polyols is that they are only partially digested and absorbed by the body in contrast to traditional sugars (i.e., glucose, maltose, and sucrose) and carbohydrates (i.e., corn syrups, maltodextrin, and starches), in turn lowering the blood glucose response (Table 2 and Livesey, 2003) and caloric density (Table 3) of foods when eaten. Caloric values reported in Table 3 represent accepted values by the respective regulatory bodies from the United States (Food and Drug Administration) and the European Union (European Food Safety Authority). Subsequently, polyols can be effective ingredients, beyond just providing application functionality, by reducing the blood glucose response and the caloric density of foods.
Polyols are not new to the food industry. Over the past 30 years, they have found many applications in various foods and confectionery products worldwide. Not only have they been used in products for diabetics for decades to help manage blood glucose levels, they have also found application to replace sugar for their physiological benefits in sugar-free candies, chewing gum, chocolate, baked goods, jams, dairy products and ice cream, among many others. The reason for their use is simple: they provide excellent and diverse functionality for the product developer to create high-quality, great-tasting, no-sugar-added, reduced-sugar, and sugar-free products.
So, why are polyols such great bulk replacers for reduced-sugar or sugar-free applications? The answer to this question becomes evident when you examine their origins. Polyols are relatively low molecular weight carbohydrates so, depending on the polyol and manufacturer, the starting point can be sucrose, glucose, fructose, lactose, xylose, or maltose from various agricultural sources. These sugars are then modified further by reducing their reactive sites (aldehyde and ketoses). By changing only the reactive groups, polyols keep much of the sugar’s structure, bulk, and function, which is what makes them an excellent 1:1 bulk replacement for sucrose.
 Range of Polyols
Range of Polyols
Polyols can essentially be divided into three groups: monomer polyols, dimer polyols, and polymeric polyol mixtures. The monomer polyols consist of erythritol, mannitol, sorbitol, and xylitol. The dimer polyols, consisting of two modified sugar units, are isomalt, lactitol, and maltitol. Lastly, the polymeric mixtures are combinations of polyols that are identified as “polyglycitol syrup”, also known as “hydrogenated starch hydrolysates” (HSH), and “maltitol syrups.” These mixtures are most similar to corn syrups. Differences between maltitol syrup and polyglycitol syrup are determined by the amount of maltitol (dry basis) present. Syrup that contains greater than 50% maltitol is considered a “maltitol syrup” and anything less is a “polyglycitol syrup” (O’Donnell, 2012).
It is commonly thought that all polyols are the same. This is far from the truth. Functional and physical properties of polyols—such as sweetness, solubility, cooling effect, or molecular weight—can differ greatly. For that reason, it is important that the formulator understand these differences to determine the best polyol option for a specific and successful product application.
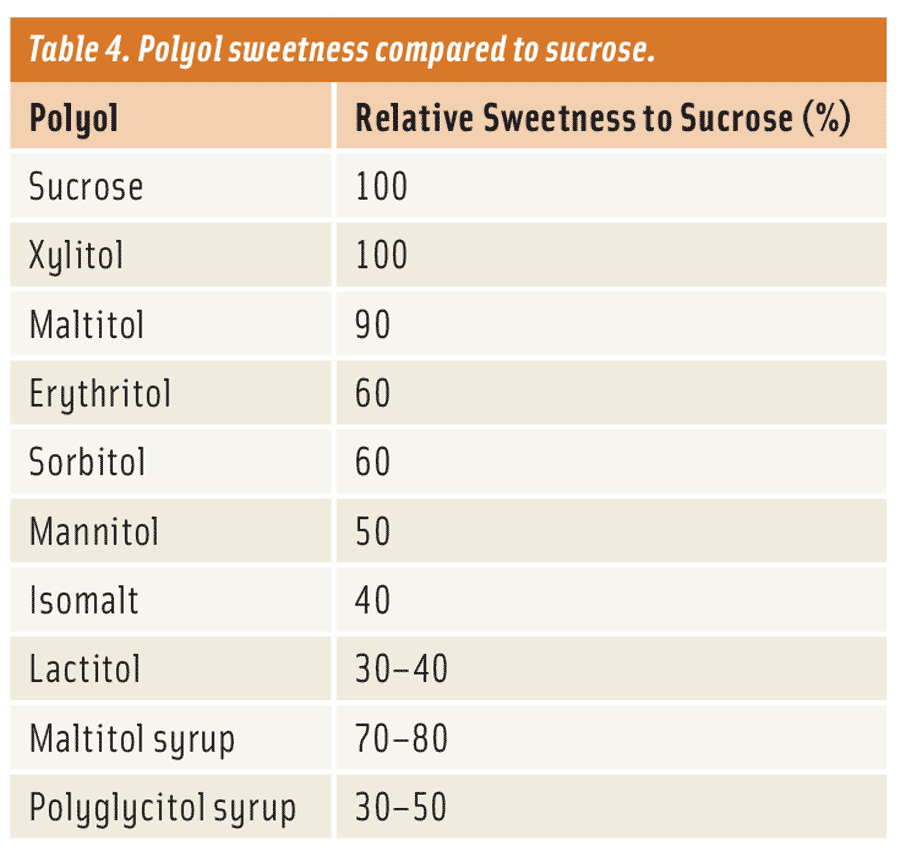
Sweetness Levels
Not surprisingly, when talking about sugar, the first thing people think about is sweetness. This is why we love sugar so much. However, when formulating reduced-sugar or sugar-free applications, one must understand that polyols have a varying range of sweetness from 0.3–1.0 times that of sucrose (Table 4). The desired level of sweetness is the easiest attribute to attain when formulating because of many effective high-potency sweeteners (HPS), such as aspartame, acesulfame potassium, stevia, and sucralose, available to provide that extra sweetening boost. In some cases, there are formulators who prefer not to use these HPS. In those instances, they may want to consider a polyol that can deliver sweetness most similar to that of sucrose or they may want to consider a lower level of sweetness with some flavors. Aside from sweetness, formulators should consider the many other important functionalities when choosing a polyol for their specific product application. There are many other important physical characteristics that need to be addressed as well to determine the most suitable polyols for the application.
--- PAGE BREAK ---
Cooling Effect
Anyone who has placed powdered sugar on their tongue has probably noticed an interesting cooling sensation. This effect, known as “heat of solution,” is an actual exchange in energy that can either lower (in this example) or raise the temperature of a solution when a substance (sugar) is added to water (saliva). The heat of solution is measured in kilojoules/kilogram (kJ/kg) and can occur if the substance interacting with water is in liquid or, in most cases, solid form. As the heat of solution decreases into more negative values, the cooling effect becomes greater and the opposite holds true for the warming effect.
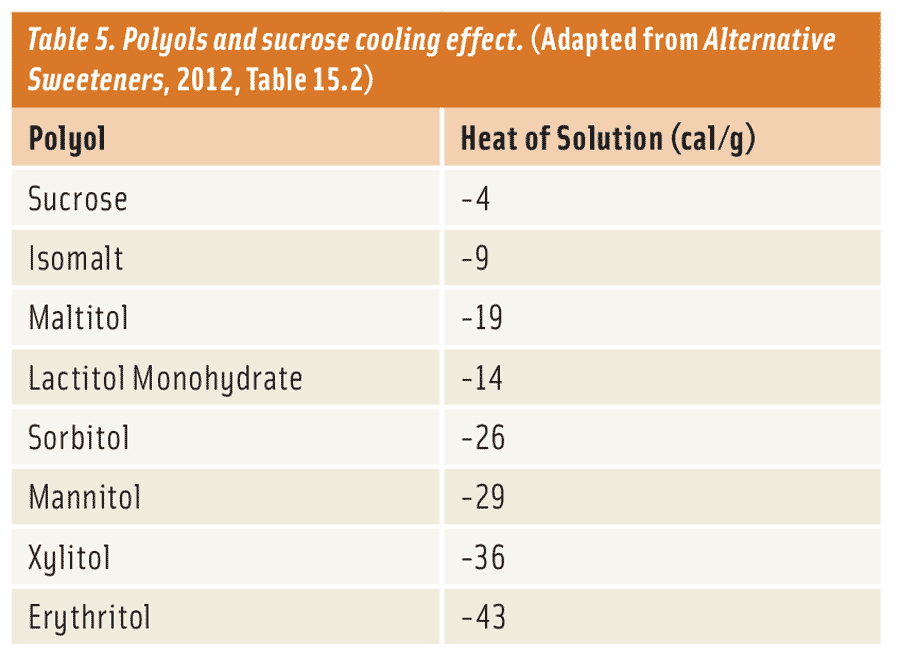
In crystalline form, most polyols tend to have a cooling effect with some being very significant. For example, erythritol has a cooling effect as high as −42 cal/g compared to only -4 cal/g for sucrose (Table 5 and Nabors, 2012). Polyols that exhibit the most cooling effect, such as erythritol, sorbitol, and xylitol, are perfect for formulators that want to deliver a strong cooling sensation for a specialty or mint-based application. There are also times when a formulator might require a minimal cooling effect for an application, such as in a snack cake or cookie. In this case, they can choose polyols such as isomalt, lactitol, and maltitol, which have a marginal cooling effect. If the formulator feels that the high-cooling polyol provides a certain functional advantage—such as cost, crystallization, solubility, or reduced calories—required to maintain product quality, but does not want a strong cooling effect, he/she can offset the cooling effect by combining it with a polyol which exhibits a weaker cooling effect. Furthermore, these high-cooling polyols can be combined effectively with other low-digestible carbohydrates like fructo-oligosaccharides, inulin, resistant maltodextrin, resistant dextrin, and polydextrose to create the same effect. These options allow for great flexibility in sugar-free and reduced-sugar applications.
Solubility
Solubility is another important aspect to be considered by the formulator when replacing traditional sugars. It is defined by the amount of a solute (sugar or bulking agent) that can be dissolved into a solvent (water) at a given temperature before becoming saturated. Typically, as the temperature of the solution increases, it can hold more of the solute. Subsequently, if a saturated solution at a higher temperature is cooled to a lower temperature, the solute will begin to drop out of solution in crystal form.
This characteristic is most important to understand if one is looking to replace sugars in traditional confectionery applications such as caramels, crèmes, marshmallows, and hard or soft candies or coatings. Confections in their most basic form are supersaturated sugar/corn syrup solutions that are either meant to be crystallized (grained) to some degree or not (ungrained).
Sweet baked goods can also experience issues if the solubility of the sugar replacer is not considered. For instance, sucrose’s solubility allows it to deliver not only sweetness but a smooth mouthfeel which can be lost in applications such as cakes or cookies if a lower soluble alternative is chosen. The shelf life can also be impacted if a polyol crystallizes too early, leading to evaporation from the baked good, causing a dry or grainy crumb or even crystallization on the product’s surface.
When trying to replace sugar, the formulator must first understand the functional requirements of the application. Is the product going to be grained? Is it going to be a low-moisture system? Are there any handling, storage, or processing concerns? What is the expected shelf life of the product? Once the formulator knows the answer to these types of questions, he/she can have a better idea of where to start.
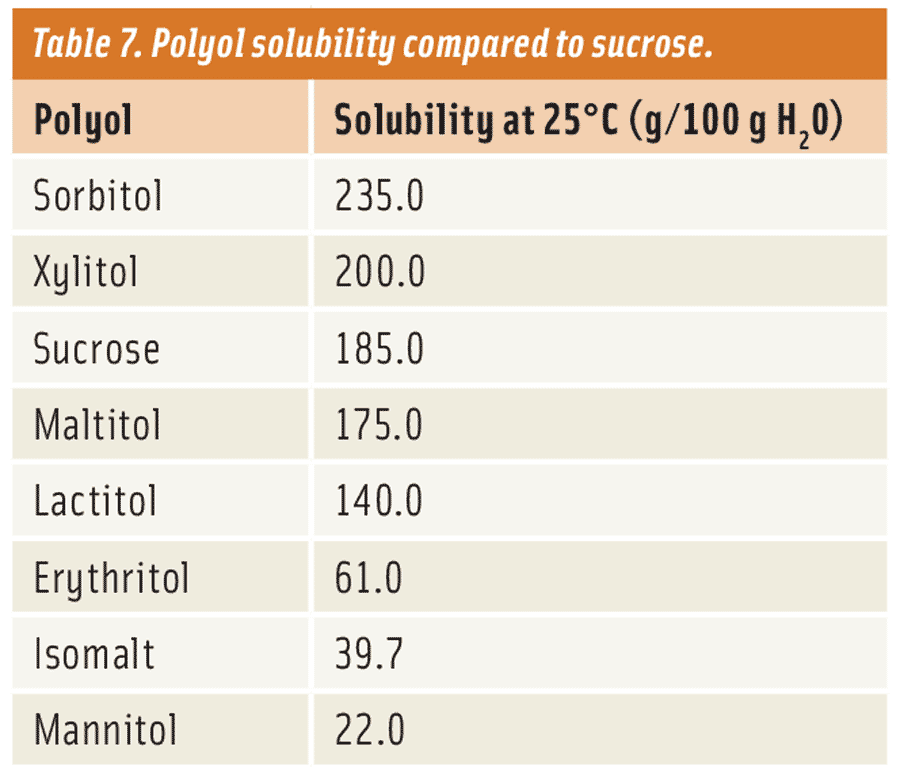
Polyols such as erythritol, isomalt, and mannitol provide the lowest solubility and crystallize most readily (see Table 7). These work well for applications that require low moisture, low hygroscopicity (ability to absorb water from surroundings), or rapid crystallization. However, formulators must be careful. For example, the solubility of mannitol is so low that it doesn’t dissolve as quickly in the mouth. The result is a chalky mouthfeel or texture.
On the other hand, lactitol, maltitol, and xylitol are more moderate in solubility and are most similar to that of sucrose. This means that their texture and flavor release will be most similar as well. This allows them to be used in more traditional applications like sucrose. Although all crystallize similarly to sucrose, lactitol seems to be the most comparable, followed by maltitol, then xylitol. In crystalline form, lactitol and maltitol are non-hygroscopic while xylitol tends to be hygroscopic. Xylitol’s tendency to pick up moisture can cause some handling issues, in the form of clumping, if not properly stored. Also, unlike the other two, xylitol’s hygroscopicity limits its use in applications that are lower in moisture like a compound coating.
One of the most soluble polyols is sorbitol. Its excellent solubility has made it an effective humectant to extend shelf life in applications such as baked goods, meat-based products, and confections.
Maltitol and polyglycitol syrups are similar to corn syrups and are very soluble for the most part. They come in a range of percent solids levels from 70–85, and are either used alone or in combination with other polyols like erythritol, isomalt, and maltitol to control crystallization. As a result, these find a wide range of use in many reduced-sugar and sugar-free applications that would traditionally use corn syrups.
--- PAGE BREAK ---
Molecular Weight
Traditional sugars/carbohydrates like glucose, fructose, maltose, sucrose, and corn syrups all have weight. The smaller molecules like the mono- and disaccharides can actually be measured. The weights are given in units of “grams per mole.” The larger molecules, consisting of many glucose units, like corn syrups, are not as easily measured because of their diverse polymeric distributions. However, there is a term commonly used to give an approximate idea of how large or small they can be. It is called “Dextrose Equivalent” (DE). During processing, corn syrups are produced through hydrolyzing starch with acid, enzymes, heat, and time to create various polymer distributions. Basically, the longer the time the corn syrup is exposed to these elements, the more those longer chains are broken down to mono- and disaccharides.
Something like this would be considered a higher DE corn syrup, with a value greater than say 43 (compared to a DE of 100—the value of glucose). This would have less viscosity, more sweetness, higher boiling points, and a lower water activity. Conversely, a DE below 43 would indicate that there was a greater presence of longer chains and less of the mono- and disaccharides. This syrup would have greater viscosity, less sweetness, more binding capability, and higher water activity.
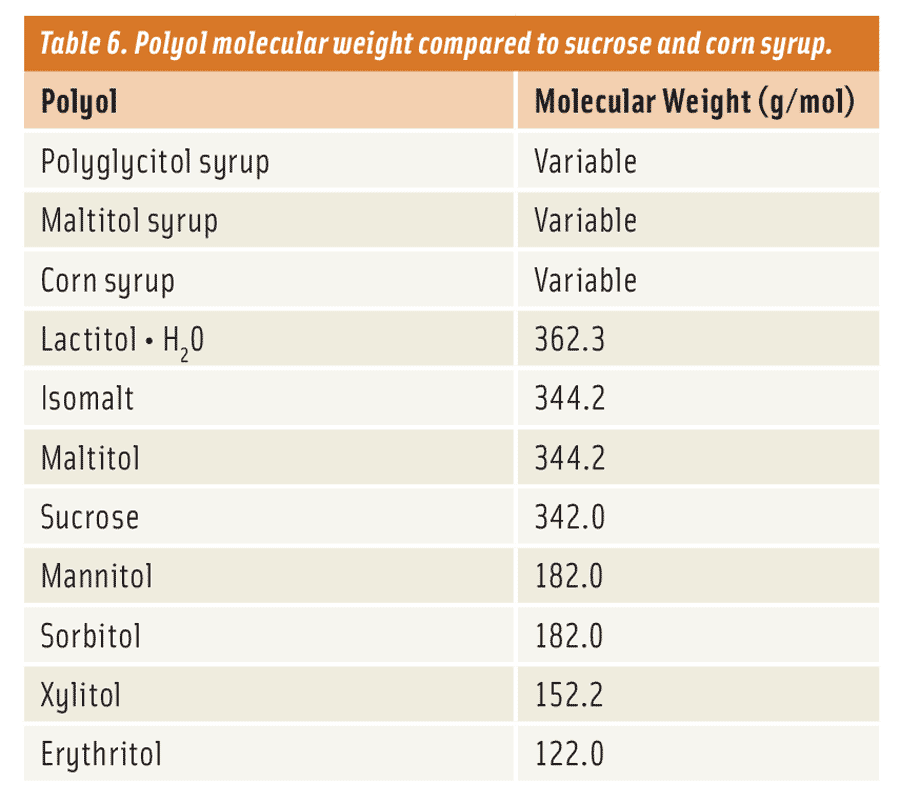
Like these traditional carbohydrates, polyols have weight (molecular weight) too and follow these similar product characteristics (Table 6). Maltitol syrups and polyglycitol syrups are most similar to the corn syrups since they are made directly from them. So in essence, they can potentially have the same colligative properties and viscosity. Typically, maltitol and polyglycitol syrups are classified by the amount of maltitol they contain without considering the rest of the carbohydrate distribution as a means of separation (although this information is available in terms of “DP” or “degrees of polymerization”). Consequently, the formulator will need to work with their supplier to understand how their maltitol and polyglycitol syrups match up in characteristics to the corresponding corn syrup they’re looking to replace.
Why is all of this important? In baked goods, for example, the molecular weight (MW) plays a significant role in dictating the overall appearance, texture, and functionality of the finished product. Why? Because the MW of the carbohydrate (sugar, corn syrup, polyol, or fiber) used in a baked good impacts the temperature at which the starch granule is hydrated during baking. This in turn causes the granule to swell, increasing the overall viscosity—due to gelatinization—of the batter or dough. It’s this phenomenon, referred to as the “starch gelatinization temperature,” that gives us the familiar spread of a cookie or volume of a cake we are so accustomed to when baking with sucrose.
As a rule of thumb, when considering sugar replacement, it’s best to examine the sugars and corn syrups that are being replaced. Then match them accordingly with the appropriate polyol or polyols. For instance, if an application were to use a 43 DE corn syrup, it would be best to consider using a polyol such as a polyglycitol or maltitol syrup of similar molecular weight instead of either a monomer or dimer polyol. For most applications, this works well or is at least a good starting point. Given that there are many different types of polyols available today that mimic traditional sugars and corn syrups, if one does their homework they can find the best fit.
 Low Digestibility
Low Digestibility
Polyols have been an important part of reduced-sugar and sugar-free products for several decades. They have proven to be great formulation tools and sugar replacers for reducing calories and lowering blood glucose responses in food products. These advantages are the result of their low digestibility or “fiber-like” physiology, e.g., like fibers, polyols are low digestible carbohydrates (Grabitske, 2008). What is not absorbed in the upper gastrointestinal tract reaches the large intestine and becomes subject to fermentation by the normal gut flora (Livesey, 2001). The resulting increase in biomass, water, and gas makes the stool softer and easier to pass. An excessive intake of polyols may eventually result in a laxative effect, causing loose stools and gas, as a normal physiological response of the colon to low-digestible carbohydrates.
Multiple Functionalities
Polyols are excellent bulk sugar replacers that have no equal when it comes to providing a wide range of functionality in reduced-sugar, no-sugar-added and sugar-free product development. Many feel that you need to replace all the sugars to have a nutritionally impactful product but this is not the case. Even a partial replacement has significant benefits and allows for more flexibility in formulating great-tasting, functionally performing products. It is important to remember that understanding the properties of polyols can be related to understanding the properties of sugars and corn syrups. Consequently, by understanding the many physical properties of each, the formulator can make an informed decision about which polyols will provide the functional aspects needed for their reduced-sugar, no-sugar-added, or sugar-free product.
Peter Jamieson is a principal at Atlas Point Technical Services LLC, 413 Stahl Ave., New Castle, DE 19720 ([email protected]).
References
Grabitske, H. and J. Slavin. 2008. “Low-Digestible Carbohydrates in Practice.” J. Am. Diet. Assoc. 108(10): 1677–1681.
Livesey, G. 2001. “Tolerance of low-digestible carbohydrates: A general view.” Br. J. Nutr. 85 Suppl. 1, S7–S16.
Livesey, G. 2003. “Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties.” Nutr. Res. Rev. 16(2): 163–191.
Nabors, L.O. 2012. Alternative Sweeteners. Fourth Ed. CRC Press.
O’Donnell, K. and M.W. Kearsley. 2012. Sweeteners and Sugar Alternatives in Food Technology. Sec. Ed. Wiley-Blackwell.
