Do Cooking Oils Present a Health Risk?
Despite the long history of cooking food in fats and oils, consumers are becoming increasingly concerned about the health effects of cooking oils. Is this concern justified, or is it fueled by a lack of understanding of the science of cooking with vegetable oils?
Article Content
The Egyptians appear to be the first to cook food using palm oil as long ago as 3000 BCE (Before the Common Era). Records also show the Greeks were deep-frying food in olive oil as early as the fifth century BCE. The use of vegetable oils from plants and fat from animals for cooking soon spread to the rest of Europe and Asia. Despite this long history of use, the vegetable oils now most commonly employed for cooking did not gain widespread use until the first half of the twentieth century. The commercial production of soybean oil in the United States began in the 1920s, and canola oil in Canada in the 1950s. By 2016 soybean, canola, corn, and palm oils accounted for almost 90% of all vegetable oils consumed in the United States (USDA 2017). Soybean oil alone amounted to 54% of all vegetable oil consumed by Americans in 2016, while soybean and canola oils together comprised 70% of the total. Palm oil is the most consumed oil worldwide. Other popular oils, such as olive, peanut, sunflower, safflower, coconut, sesame, avocado, and grape-seed oils, make up only a few percent of all the vegetable oils used in cooking and salads.
Since 2003 consumption of all vegetable oils has increased by 157% in the United States. Consumers have become increasingly concerned with the safety of cooking oils. In the last two years The Nutrition Source digital newsletter published by the Harvard T.H. Chan School of Public Health has received numerous comments and inquiries about the safety of cooking oils. This is supported by a Google Trends search of the words “cooking oil bad,” which shows that interest in this topic has doubled from 2008 to 2016 (Crosby 2018).
When cooking oils are subjected to heat in the presence of air and water (from food), such as in deep-fat frying and sautéing (pan frying), they can undergo at least three chemical changes: 1) oxidation of the fatty acids, 2) polymerization of the fatty acids, and 3) breaking apart of the triglyceride molecules into free fatty acids and glycerol by hydrolysis (reaction with water from the food being cooked) (Choe and Min 2007). All three chemical changes increase with cooking time and temperature and are accelerated by the presence of food. When it comes to the health aspects of cooking with vegetable oils, oxidation of the fatty acids is very important. During cooking, oxidation of fatty acids, both free and in triglycerides, produces very small amounts of dozens of new compounds called aldehydes, ketones, and alcohols. These compounds produce the wonderful flavors of fried foods. But in sufficient quantities, some of these compounds can be toxic.
Oxidation of Cooking Oils
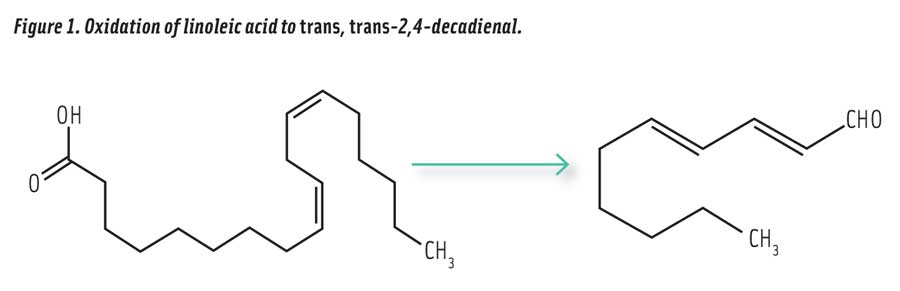
Consider the example of trans, trans-2,4-decadienal (the most stable isomer, referred to as 2,4-decadienal), a volatile aldehyde produced in vegetable oils by the oxidation of linoleic acid (Figure 1) that is responsible for much of the enticing aroma of fried food. This unsaturated aldehyde is formed in heated soybean oil in far higher amounts than any other aldehyde (Zhang et al. 2015). The U.S. National Toxicology Program has compiled an extensive report on the toxicity of 2,4-decadienal (NIH 2011). The oral LD50 (lethal dose for 50% of test animals) in rats is >5000 mg/kg. In rats and mice, this compound has been found to have a “no adverse effect level” of 100 mg/kg of body weight. This would be equivalent to no adverse effects after ingestion of up to 7 g by a 70 kg human, which is considered very low toxicity. Compare this with the no adverse effect level reported for caffeine at only 3–5 mg/kg of body weight (equivalent to 3 cups of coffee) (Stavric 1998). Above the no effect level, 2,4-decadienal begins to show biological effects in rats and mice, including decreased body weight and stomach lesions after three months of oral feeding administered by gavage at levels up to 800 mg/kg. The compound is not mutagenic by in vitro or in vivo tests using six different strains of Salmonella typhimurium (NIH 2011), but has been reported to be cytotoxic and genotoxic, as well as promote the oxidation of LDL cholesterol (Boskou et al. 2006). Although the compound exhibits low toxicity, it is desirable to limit exposure to 2,4-decadienal because of the relatively high levels formed in oxidized cooking oils.
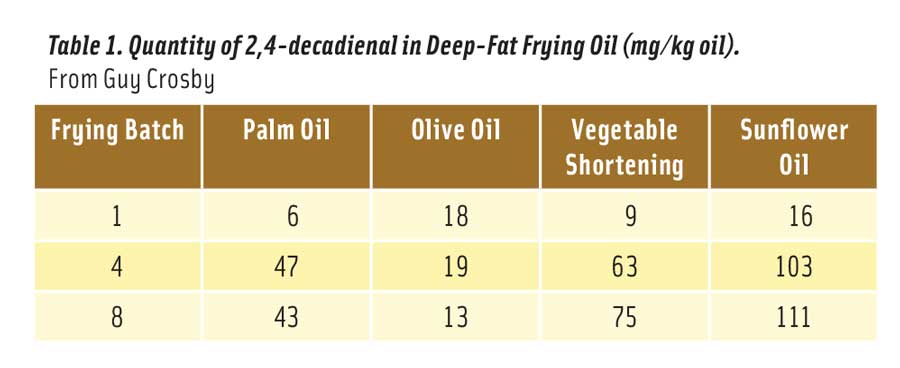
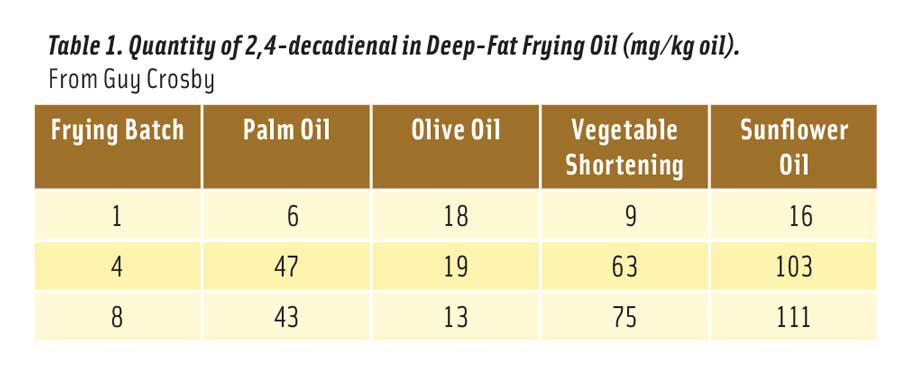
But how much 2,4-decadienal is produced in heated vegetable oil? It depends on the oil, the temperature of the oil, and how long the oil was heated at this temperature. The optimum temperature for frying food is considered to be 180°C (356°F) (Katragadda et al. 2010). Table 1 shows the mg of 2,4-decadienal formed in 1 kg of frying oil after 1, 4, and 8 consecutive fryings for 8–9 minutes each at 175°C (347°F) (Boskou et al. 2006). The reason that palm and olive oils contained less of the aldehyde after 8 fryings is because the smaller amount of linoleic acid in these oils declined due to oxidation after 4 fryings.
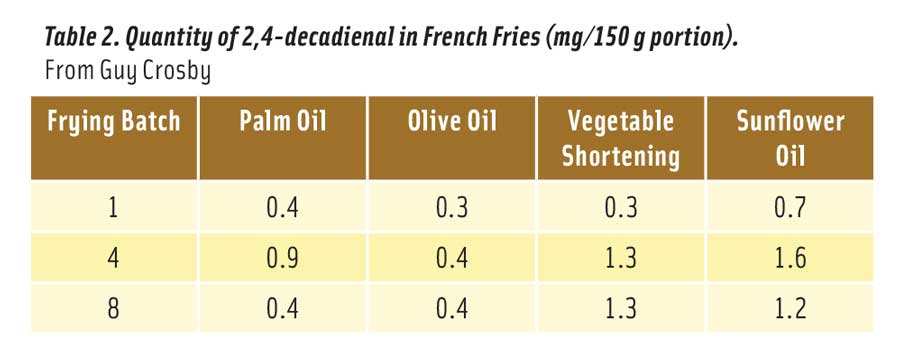
Table 2 shows the amount of 2,4-decadienal absorbed into a single 150 g portion of french fries cooked in the oils after 1, 4, and 8 fryings (Boskou et al. 2006). Note that “vegetable shortening” was a commercial blend of sunflower, palm, and cottonseed oils. The levels of 2,4-decadienal were all well below the no effect level of 100 mg/kg. Relative to sunflower oil, french fries cooked in regular olive oil absorbed 70% less 2,4-decadienal, while fries cooked in palm oil absorbed 43% less of the aldehyde. Sunflower oil is more unsaturated than either olive or palm oils and is more readily oxidized to 2,4-decadienal.
Fatty acids with higher levels of unsaturation are oxidized more rapidly during heating in air. For example, linoleic acid containing two double bonds is oxidized about 12 times faster than monounsaturated oleic acid, while linolenic acid, containing three double bonds, is oxidized about 25 times faster (Belitz et al. 2009). Canola oil heated at 180°C produces about 3.4 times more 2,4-decadienal than regular olive oil (Fullana et al. 2004). Olive oil is much higher in monounsaturated oleic acid (79% of the fatty acids) than canola oil (61%), which also contains much more polyunsaturated linoleic acid (21%) than olive oil (6.3%) (Stauffer 1996). In other words, canola oil is more unsaturated (contains more carbon-carbon double bonds) than olive oil, so it is oxidized much more readily (Crosby 2015). Similarly, soybean oil with even more linoleic acid (54%) produces almost 4.5 times more 2,4-decadienal than olive oil. Higher levels of unsaturation mean more oxidation during frying, so canola and soybean oils produce significantly more 2,4-decadienal than olive and palm oils.
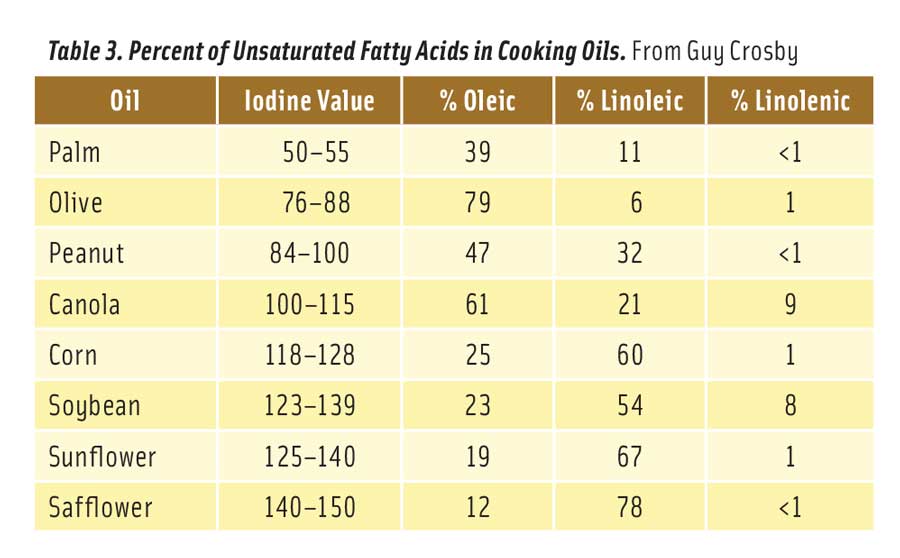
Table 3 shows the level of unsaturation of selected vegetable oils. The iodine value is an old but reliable measure of the number of double bonds in a fat or oil. A higher value means more double bonds (Stauffer 1996). Table 3 also compares the levels of oleic, linoleic, and linolenic acids in these oils (Stauffer 1996). The oils are arranged in order of unsaturation, with the most unsaturated on the bottom of the table. To reduce the formation of 2,4-decadienal, cooks should select oils from near the top of the table.
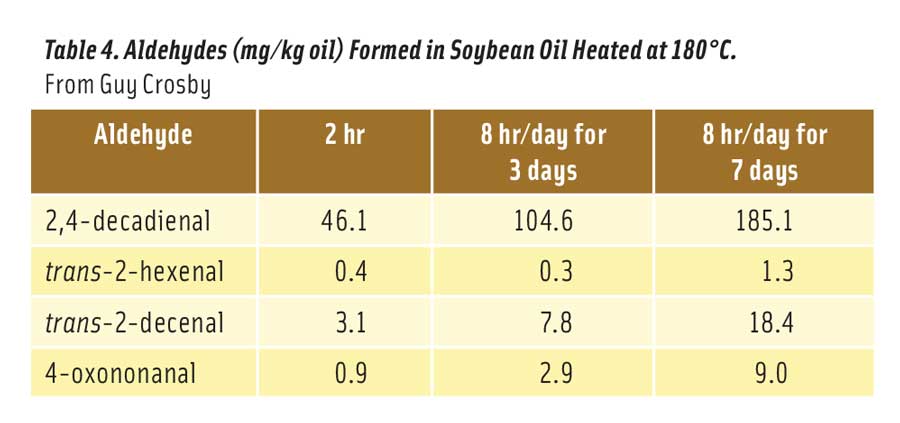
More than two dozen different aldehydes have been identified in heated vegetable oils (Zhang et al. 2015). Most are quite innocuous at the levels produced in oil, while others like 2,4-decadienal may exhibit low levels of toxicity depending on the quantity produced. Based on material safety data sheets, oral LD50s for these aldehydes in rats range from a low of about 780 mg/kg (trans-2-hexenal) to >5000 mg/kg for many of the aldehydes. Table 4 shows the levels of four potentially toxic aldehydes formed in soybean oil after heating at 180°C for 2 hr, 3 days for 8 hr/day, and 7 days for 8 hr/day (Zhang et al. 2015). As noted here, 2,4-decadienal is formed at much higher levels than the other aldehydes, especially after heating the oil for many hours.
Thermal Degradation of Cooking Oils
One especially toxic aldehyde, acrolein, is produced when vegetable oils are heated to their smoking point (the temperature at which an oil starts to smoke). It is not a product of oxidation like the other aldehydes. At this high temperature, the triglycerides begin to break down to free fatty acids and glycerol, and the glycerol is quickly dehydrated to acrolein. The blue haze seen above smoking oil is caused by traces of acrolein (Katragadda et al. 2010). Acrolein can be formed from glycerol at temperatures as low as 180°C (356°F) (Katragadda et al. 2010). Not only does acrolein have a very pungent, irritating smell, it is quite toxic with an oral LD50 in rats of only 46 mg/kg of body weight. Although acrolein has not been classified as to carcinogenicity, the U.S. Occupational Safety and Health Administration has set a workplace limit in air at 0.1 parts per million. Fortunately, acrolein is so volatile (boiling point, 52°C/125°F) that very little, if any, remains in cooking oil.
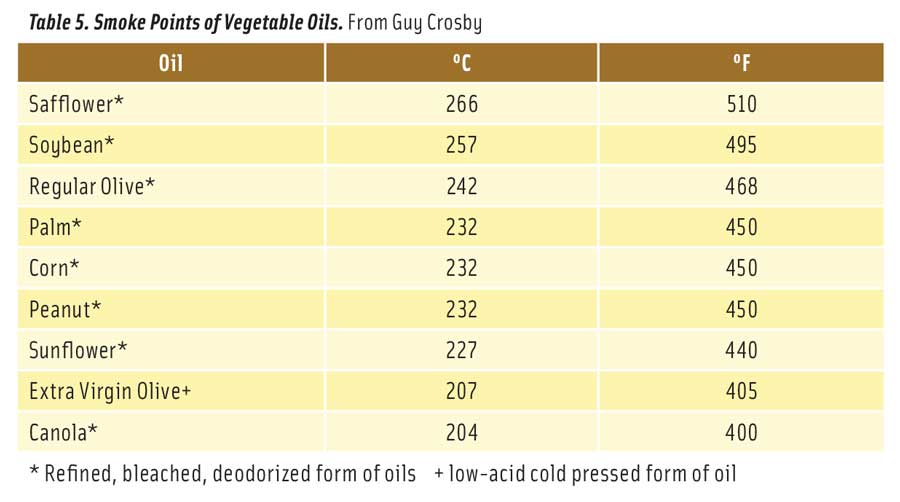
Table 5 lists the smoke points of fresh vegetable oils (Chu 2004). There is significant variation in reported smoke points of oils. In general, it is best to select oils with high smoke points. But at the same time, it is also best not to overheat any vegetable oil. After just a single exposure to frying temperatures, vegetable oils begin to break down to free fatty acids and glycerol. As free fatty acids increase in the oil, its smoke point declines rapidly. The longer oil is used for frying, the lower its smoke point. For example, the formation of as little as 1% free fatty acid in soybean oil causes a 25% decline in its smoke point (Stauffer 1996). Some European countries set a lower limit for the smoke point of used frying oils at 170°C (338°F). At this point, the oil contains about 0.7% free fatty acids and should be discarded (Belitz et al. 2009).
 Prolonged Heating of Cooking Oils
Prolonged Heating of Cooking Oils
Particles of fried food building up in oil during repeated frying operations dramatically lower the smoke point of the frying oil. For this reason, restaurants and foodservice establishments typically filter their frying oil every day, but often don’t change the oil more than a few times per week. A recent survey of frying oils used in independent (nonfranchise) restaurants found that about 35% exceeded acceptable in-use oil quality (Sebastian et al. 2014). As shown in Table 4, heating oils for long periods of time between changes significantly increases the level of aldehydes in the oil. Prolonged heating also produces small amounts of trans fatty acids (Crosby 2015). However, oil turnover and replenishment of oil absorbed by the fried food with fresh makeup oil reduces the buildup of oxidation products and trans fats. Generally, oil quality cannot be maintained in batch fryers with a turnover rate of greater than or equal to 20 hours of operation (Dunford 2017). The best restaurants employ simple tests to determine when it is time to change the oil, fry at 177°C (350°F), and change oil three or more times per week (Crosby 2017). In addition to fried foods consumed away from home, many consumers are purchasing frozen fried foods at supermarkets because of their convenience and appeal. Most of these products are manufactured in continuous frying operations using more saturated oils, such as palm oil, and mechanical filtration. In a typical doughnut process, the oil is turned over about every 8 hr due to absorption of the oil by the doughnuts. These conditions tend to reduce oxidation and degradation of the oil relative to batch fryers in restaurants and foodservice establishments that may use the same oil for much longer periods (Fellows 2009).
 Health Concerns
Health Concerns
Among those with a concern for the safety of cooking food in vegetable oils, the assessment of risk is still unresolved. Some feel the oxidation products are significant risk factors for human health (Kanner 2007), while others believe levels of natural antioxidants and food detoxifying enzymes in our body provide protection against the harmful effects of oxidized oil (Baynes 2007). Clearly, more information is needed to resolve this important public health concern (Monnier 2007, Chiou and Kalogeropoulos 2017). In the meantime, what are some helpful recommendations for consumers and foodservice operators? First of all, cooking with vegetable oil in the home (frying or sautéing) presents very little risk as the oil is typically used only once and heated for a short period of time (often less than 10 min). It is also possible to control the temperature of the oil, which should not exceed 190°C (374°F) and is best heated to 180°C (356°F) or lower. The oil should also not be heated at or above the oil’s smoke point. For home use, any of the oils listed in Table 5 are acceptable, although those that are less unsaturated are preferred to reduce oxidation.
The risk does not come from cooking with vegetable oil in the home, but in excessive consumption of fried food, especially outside of the home in restaurants and foodservice establishments where the oil may be heated for long periods before changing, as well as from the increasing consumption of fried foods from supermarkets. The evidence suggests there is a significantly higher risk of developing a number of chronic diseases, such as type 2 diabetes, heart failure, obesity, hypertension, and coronary artery disease, when fried foods are consumed four or more times per week, especially fried food consumed outside of the home (Cahill et al. 2014, Gadiraju et al. 2015). Presently there is insufficient evidence to determine if there is any correlation between these chronic diseases and the type of oil or food, temperature and duration of frying, or how long the oil was heated before changing.
Conclusions
The technology of cooking oils is changing rapidly. At one time, partially hydrogenated vegetable oils were thought to be the answer as these highly saturated fats provided good stability to oxidation. But then it was realized that the very high levels of trans fats in these oils was a cause of cardiovascular disease. Today very little partially hydrogenated vegetable oil is still in use, and the U.S. Food and Drug Administration requires that the level of trans fat be declared on food labels (Crosby 2015). In response, manufacturers of cooking oils are developing new blends of oils with better stability toward oxidation and virtually no trans fat.
Vegetable oils with increased levels of oleic acid and reduced levels of linoleic and linolenic acids have been developed through traditional hybridization or genetic engineering of the oil-producing plants. For example, soybean, canola, sunflower, and safflower oils with 75%–90% less linoleic acid (replaced with higher levels of oleic acid) are available. And the use of more saturated palm oil for frying has increased dramatically. Unfortunately, many of these newer oils are not available for home use. So consumers should be aware that olive oil is the best choice for frying and sautéing food in the home. It is more stable to oxidation than most other oils, is high in natural antioxidants, and regular olive oil has a high smoke point. Compared with newer oils, it has a very long history of use for cooking food, especially as part of the healthy Mediterranean diet (Chiou and Kalogeropoulos 2017).
Guy Crosby, PhD, CFS, a member of IFT, is adjunct associate professor, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston ([email protected]).