Food Allergies and Other Food Sensitivities
A publication of the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition
Centuries ago, Lucretius said that one man’s food may be another man’s poison. Such is the case with food allergies. Individuals with food allergies and other types of food sensitivities react adversely to the ingestion of foods and food ingredients that most consumers can ingest with no problems.
Eating is necessary to sustain life. For most consumers, eating is an enjoyable experience given the variety and abundance of foods available in the marketplace. For some individuals, however, consuming certain foods can be a debilitating, possibly even life-threatening, experience. Individuals with various forms of food allergies and sensitivities must avoid certain foods or food ingredients in their diets. For such people, the joy of eating is diminished by the ever-present concern that they might consume a food or food ingredient that will elicit an adverse reaction. For them, food selection can become a tedious task requiring the painstaking reading of ingredient lists on the labels of packaged foods and a ceaseless search for more knowledge about food composition. Food preparation requires careful attention to details such as “cooking from scratch,” seeking alternative recipes for many dishes, and avoidance of shared utensils, containers, and cooking surfaces between allergenic and non-allergenic foods. In situations where one family member has a very serious allergic sensitivity, the entire family often has to avoid the offending food as a precautionary measure.
Definitions
Food allergies and other food sensitivities are individualistic adverse reactions to foods (Taylor, 1987). These food-related illnesses are individualistic because they affect only a few people in the population; most consumers can eat the same foods with no ill effects. Many different types of reactions are involved in these individualistic adverse reactions to foods (Fig. 1; Anderson, 1996; Taylor, 1987). Adverse food reactions can include IgE and non-IgE-mediated primary immunological sensitivities, non-immunological food intolerances, and secondary sensitivities. While these various types of reactions are often considered collectively as food allergies, true food allergies represent only a fraction of the individualistic adverse reactions to foods.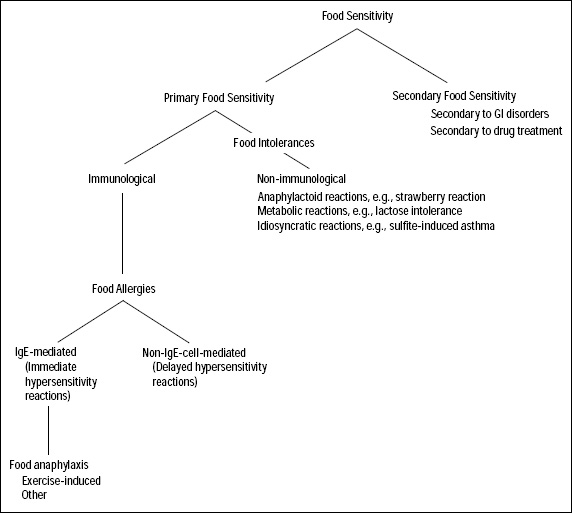
True Food Allergies. True food allergies are abnormal (heightened) responses of the immune system to components of certain foods (Lemke and Taylor, 1994). The components of foods that elicit these abnormal immune responses are typically naturally-occurring proteins in the foods (Bush and Hefle, 1996). True food allergies can be divided into two categories based upon the nature of the immune response—immediate hypersensitivity reactions and delayed hypersensitivity reactions (Lemke and Taylor, 1994). In immediate hypersensitivity reactions, symptoms begin to develop within minutes to an hour or so after ingestion of the offending food. Immediate hypersensitivity reactions have been noted with many foods and can sometimes be quite severe (Hefle et al., 1996). Immediate hypersensitivity reactions involve abnormal responses of the humoral immune system with the formation of allergen-specific immunoglobulin E (IgE) antibodies (Mekori, 1996). In delayed hypersensitivity reactions, symptoms do not begin to appear until 24 hours or longer after the ingestion of the offending food (Lemke and Taylor, 1994). With the exception of celiac disease, which involves an abnormal immunological response to wheat and related grains (Ferguson, 1997), the role of delayed hypersensitivity reactions to foods remains poorly defined. Delayed hypersensitivity reactions involve abnormal responses of the cellular immune system with the development of sensitized T cells (Lemke and Taylor, 1994).
Allergy-Like Intoxications. Certain foods can elicit adverse reactions that resemble true food allergies. These foods contain elevated levels of histamine, one of the principal mediators of allergic reactions in the body (Taylor et al., 1989a). In true food allergies and anaphylactoid reactions, however, histamine is released from intracellular locations (Mekori, 1996). In these allergy-like intoxications, histamine is ingested with foods. Histamine poisoning is often known as scombroid fish poisoning because it is frequently associated with consumption of spoiled fish of the scombroid type such as tuna and mackerel (Taylor et al., 1989a). However, histamine poisoning can also occur from the ingestion of spoiled fish such as mahi-mahi and bluefish that are not scombroid fishes (Etkind et al., 1987; Taylor et al., 1989a). And, histamine poisoning has even been occasionally associated with the ingestion of cheese, especially aged Swiss cheese (Stratton et al., 1991; Taylor et al., 1982). In all of these food products, specific types of bacteria have proliferated and caused the conversion of the amino acid, histidine, into histamine (Stratton and Taylor, 1991; Sumner et al., 1985; Taylor et al., 1978). Ingestion of small amounts of histamine in the diet is a normal occurrence and does not cause any harm. However, when large doses of histamine are ingested with foods, the body’s protective mechanisms can be overwhelmed resulting in histamine poisoning (Taylor, 1986). Unlike food allergies and sensitivities, all consumers are susceptible to histamine poisoning. Because this illness is not truly a form of food allergy or sensitivity, it will not be discussed further. It does merit some mention because the similarity in symptoms can cause it to be confused with true food allergy.
--- PAGE BREAK ---
Food Intolerances. Food intolerances are abnormal reactions to foods or food components that do not involve the immune system. Several different types of food intolerances are also known to exist. These intolerances are metabolic food disorders, anaphylactoid reactions, and idiosyncratic reactions (Taylor, 1987). Metabolic food disorders are adverse reactions to a food or food component that results from a defect in the metabolism of these foods or some substance therein or from an affect of the food or food component on the body’s normal metabolic processes. Lactose intolerance is an example of a metabolic food disorder resulting from a defect in the metabolism of a food component (Kocian, 1988). Favism is an example of a metabolic food disorder resulting from foodborne substances that interfere with normal metabolic processes (Mager et al., 1980). Anaphylactoid reactions are adverse reactions resulting from the ingestion of foodborne substances that release histamine from cellular stores within the body (Taylor, 1987). There are no particularly good examples of anaphylactoid reactions, although circumstantial evidence suggests that such reactions may occur. Food idiosyncrasies are adverse reactions to foods or food components that occur through unknown mechanisms and which can even include psychosomatic illnesses (Taylor, 1987). Sulfite-induced asthma is the best example of an idiosyncratic reaction that has been well documented to occur among certain consumers, although the mechanism remains unknown (Bush and Taylor, 1998).
Secondary Food Sensitivities. Adverse reactions to foods or food components can occur with or after the effects of other conditions. Examples of such reactions, termed secondary food sensitivities, include lactose intolerance secondary to gastrointestinal disorders such as Crohn’s disease or ulcerative colitis (Metcalfe, 1984a) and drug-induced sensitivities such as the increased sensitivity to tyramine among patients on monoamine oxidase-inhibiting drugs (Blackwell and Marley, 1969).
IgE-Mediated Food Allergies
This type of food allergy can also be called immediate hypersensitivity, Type I allergy, or food anaphylaxis. The Greek word, anaphylaxis, means “against protection” and refers to allergic reactions to foreign protein molecules. IgE is one of five classes of antibodies that are present in the human body and that play a role in disease resistance. IgE antibodies are particularly involved in fighting off parasitic infections. Although all humans have low levels of IgE antibodies, only individuals predisposed to the development of allergies produce IgE antibodies that are specific for and recognize certain environmental antigens. These antigens are typically proteins, although only a few of the many proteins in nature are capable of stimulating the production of specific IgE antibodies in susceptible individuals (Taylor, 1996). The allergens eliciting IgE antibody formation can be found in pollens, mold spores, bee venoms, dust mites, and animal danders in addition to foods (Solomon and Platts-Mills, 1998).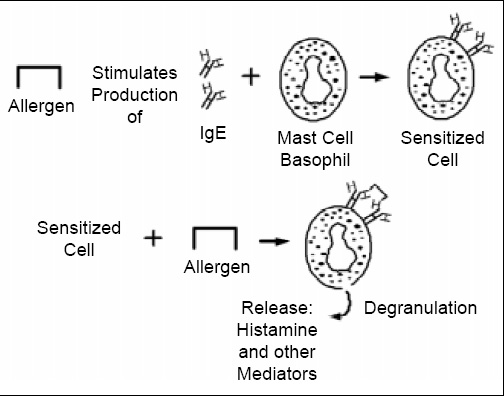
Pathogenesis. The mechanism of IgE-mediated allergic reactions is depicted in Fig. 2. First, sensitization by the allergen must occur. In the sensitization phase of the response, the allergen stimulates production of specific IgE antibodies. While sensitization can occur with the first exposure to the allergen, that is not always the case. With respect to food allergens, sensitization does occur most commonly among young infants where the immune response seems to be more likely to be oriented toward an IgE response (Anderson, 1996). However, even in susceptible infants exposure to most dietary proteins results in oral tolerance, a normal immunologic response that is not associated with adverse reactions, rather than sensitization (Strobel, 1997). The specific IgE antibodies then attach to mast cells in various tissues and basophils in the blood. Mast cells and basophils contain granules that are loaded with physiologically active chemicals that mediate the allergic response (Church et al., 1998). On subsequent exposure to the allergenic substance, the allergen cross-links two IgE antibodies on the surface of the mast cell or basophil membrane, stimulating the release into tissues and blood of a host of allergic response mediators. Although many mediators have been described, histamine is one of the primary mediators responsible for many of the immediate symptoms that occur in IgE-mediated allergic reactions (Simons, 1998). Other important mediators include the various leukotrienes and prostaglandins, some of which are associated with more delayed symptoms that can occur in IgE-mediated, immediate hypersensitivity reactions, the so-called late-phase responses (Peters et al., 1998).
--- PAGE BREAK ---
Symptoms. IgE-mediated food allergies are associated with a wide variety of symptoms, ranging from mild and annoying to severe and life-threatening (Lemke and Taylor, 1994). The symptoms can involve the gastrointestinal tract, skin, or respiratory tract (Table 1). Food-allergic individuals usually suffer from only a few of the many possible symptoms. The nature and severity of the symptoms experienced by a food-allergic individual may also vary from one episode to the next depending on the dose of the offending food that has been inadvertently ingested, the degree of sensitization to the offending food at the time of the episode, and probably other factors. Because foods are ingested, gastrointestinal symptoms are often encountered (Anderson, 1996; Gryboski, 1991). However, these symptoms can also be involved in other illnesses so their association with food allergies is often difficult to decipher. Cutaneous symptoms such as urticaria (hives) and dermatitis (eczema) are also common manifestations of food allergies (Kaplan, 1998). These symptoms are more definitive for food allergy, although the frequency of dermatitis as a manifestation of food allergy especially, in infants, has only been widely appreciated in the past decade or so (Sampson, 1988). Respiratory symptoms are less commonly associated with food allergies than with environmental allergies such as pollen or animal dander allergies (Taylor et al., 1999). With environmental allergies, the allergens are inhaled, so the primary involvement of respiratory symptoms is understandable. However, ingested food allergens must survive digestive processes and be absorbed to elicit systemic reactions in the respiratory tract. Although few asthmatic individuals experience food-induced asthma, asthma is among the most severe symptoms associated with food allergies (Bousquet and Michel, 1988). Food-induced asthma is a risk factor for severe, life-threatening reactions to the offending foods (Sampson et al., 1992).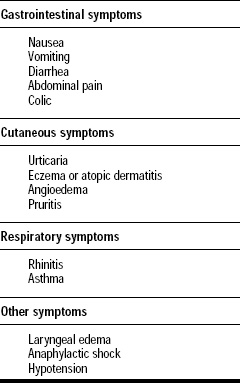
Oral allergy syndrome is perhaps the most common manifestation of food allergy (Ortolani et al., 1988). Oral allergy syndrome is often so mild that it is ignored by afflicted individuals. Oral allergy syndrome involves symptoms confined to the oropharyngeal area including hives, itching, and swelling (Pastorello and Ortolani, 1997). Fresh fruits and vegetables are the foods most frequently associated with oral allergy syndrome (Pastorello and Ortolani, 1997). Individuals with oral allergy syndrome are usually sensitized to one or more pollens, and react to proteins in specific fresh fruits and vegetables that cross-react with the pollen allergens (Calkoven et al., 1987; Ebner et al., 1995; van Ree and Aalberse, 1993). Examples would include allergic reactions to watermelons and other melons in ragweed-allergic individuals (Enberg et al., 1987), allergic reactions to celery in mugwort-allergic or birch-sensitized individuals (Ballmer-Weber et al., 2000; Wuthrich et al., 1990), and allergic reactions to apples and hazelnuts in birch-allergic individuals (Dreborg, 1988). Because the allergens in these foods are inactivated on contact with stomach acid and digestive proteases, systemic reactions to these fresh fruits and vegetables are rarely encountered (Taylor and Lehrer, 1996). Because these allergens are denatured by heating (Dreborg and Foucard, 1983; Taylor and Lehrer, 1996), individuals with oral allergy syndrome can usually safely ingest heat-processed forms of the offending food, e.g., apple jelly or apple sauce. Recent evidence indicates that, in contrast to previous assumptions, some individuals with oral allergy syndrome associated with certain foods, such as celery, also may experience more severe systemic reactions on occasion (Ballmer-Weber et al., 2000).
The most frightening symptom associated with food allergies is anaphylactic shock, which usually involves multiple systems including the gastrointestinal tract, the skin, the respiratory tract, and the cardiovascular system. Symptoms occur in combination and develop rapidly. Severe hypotension can occur. Death can occur from cardiovascular and/or respiratory collapse within minutes of ingestion of the offending food. Only a few of the many people with IgE-mediated food allergies are at risk for such serious manifestations. However, numerous deaths have been attributed to inadvertent exposure to the offending food among individuals with food allergies (Bock et al., 2001; Sampson et al., 1992; Yunginger et al., 1988). These deaths have involved asthma and/or anaphylactic shock.
In addition to allergic reactions associated with the consumption of foods, occupational food allergies including occupational asthma, hypersensitivity pneumonitis (extrinsic alveolitis), and contact dermatitis can occur amongst food industry employees (O’Neil and Lehrer, 1997). Such reactions can be triggered in food manufacturing workers by food-derived protein allergens, e.g., green coffee beans, flour, and shellfish, or non-food agents, e.g., honey bees and latex products (Lehrer and O’Neil, 1992). These occupational allergies are produced by respiratory exposure to dusty processing environments or cutaneous exposure to specific food products.
--- PAGE BREAK ---
Diagnosis. With the wide range of symptoms that can be involved in IgE-mediated food allergies and the possibility of other causes for many of these symptoms, the diagnosis of IgE-mediated food allergies can sometimes be challenging (Metcalfe, 1984b). First and foremost, an association must be sought between the ingestion of one or more offending foods and the elicitation of the adverse reaction. Once a food-associated adverse reaction is well documented, then proof of the existence of an IgE mechanism must be considered. The assistance of an allergist should be sought in the diagnosis of IgE-mediated food allergies. Too often, consumers rely upon self-diagnosis or parental diagnosis. Self-diagnosis and parental diagnosis of reactions in children are problematic because they are often erroneous, leading to the identification of the wrong foods, and implicate too many foods (Bock et al., 1978). Careful history-taking including the use of food diaries by an experienced allergist can often identify suspect foods. Elimination diets followed by challenges can sometimes confirm the existence of a food-associated adverse reaction. However, the gold standard for documenting existence of a food-associated adverse reaction is the double-blind, placebo-controlled food challenge (DBPCFC; Bock et al., 1988). Such clinical challenges are especially useful in situations where the role of a specific food remains somewhat questionable. Double-blind, placebo-controlled food challenges are not usually done in cases involving serious, life-threatening adverse reactions because of the obvious risks to the patient and the likelihood in such cases that the role of one or more specific foods is rather clear. Once the role of one or more specific foods in the adverse reaction has been established, then the involvement of the IgE mechanism can be documented through skin-prick tests using extracts of the suspect foods (Bock et al., 1977) or by radioallergosorbent tests (RASTs) where the presence of food-specific IgE antibodies in the blood serum is examined (Adolphson et al., 1986).
Prevalence. IgE-mediated food allergies likely affect between 2 and 2.5% of the total population. For many years, the overall prevalence estimate for IgE-mediated food allergies has been between 1 and 2% of the total population (Lemke and Taylor, 1994). However, a recent random, digit-dial telephone survey in the U.S. indicated that the combined prevalence of peanut and tree nut allergies was an estimated 1.14% (Sicherer et al., 1999). And, a similar telephone survey in the United Kingdom indicated that the estimated prevalence of peanut allergy alone was 0.5% (Emmett et al., 1999). While one could criticize the use of telephone surveys, it is unlikely that many consumers would mis-diagnose peanut or tree nut allergies since the symptoms are profound and usually quite immediate. Thus, these surveys may be reasonably reliable and a good indication that previous estimates based solely on clinical impression were incorrect. Certainly, if peanut and tree nut allergy alone account for more than 1% of IgE-mediated food allergy, then the overall prevalence of food allergy in the total population likely exceeds 2%.
Infants (1–3 years of age) and children are more commonly affected by food allergies than other age groups (Taylor et al., 1999). Among infants younger than 3 years, the prevalence of food allergies appears to be in the range of 5% to 8% (Sampson, 1990). The prevalence of food allergy among young infants (<1 year of age) has been studied more thoroughly than has the prevalence among older children and adults.
A much higher proportion of the public believes that they have food allergies because of self-diagnosis, parental diagnosis, and misconceptions about the definition of food allergy even among some physicians (Bock et al., 1978; Sloan, 1986). Studies have shown that 10 to 20% of the consuming public believes that they or someone in their family has a food allergy (Chiaramonte et al., 1999; Sloan, 1986).
Some believe, on the basis of the impressions of clinicians involved in allergy practice for several decades, that the prevalence of IgE-mediated food allergies is increasing. An increase in prevalence, however, is difficult to confirm because good baseline data from earlier years for comparative purposes are lacking. Certainly, the awareness of food allergy has increased. More individuals may seek specialized medical attention from allergists as a result of this increased awareness. But, almost everyone agrees that the prevalence of severe food allergies seems to be increasing. The reasons for this apparent increase are unknown. Many severely affected individuals have food-induced asthma as one of the manifestations of their allergic reaction. The overall prevalence of asthma is definitely increasing in the U.S. for unknown reasons (Beasley et al., 2000). While food-induced asthma is a small fraction of the total asthma population, the prevalence of food-induced asthma may be increasing in concert with the overall increase in prevalence of asthma.
Most Common Allergenic Foods.
The prevalence of allergies to specific foods is not precisely known. Cows’ milk allergy appears to be among the more prevalent food allergies in infants. This is not surprising given the importance of milk in infant feeding practices. The prevalence of cows’ milk allergy among infants under the age of two in Sweden, Denmark, and Australia has been studied and found to be approximately 2% in all three countries in well-conducted clinical studies involving groups of unselected infants followed from birth to the age of two years (Hill et al., 1997; Host and Halken, 1990; Jakobsson and Lindberg, 1979). The prevalence of milk allergy is known to diminish with age (Bock, 1982), but the exact prevalence of milk allergy among other age groups is unknown. The prevalence of other specific food allergies has not been established in controlled clinical trials using unselected population groups. The comparative prevalence of various specific food allergies can be discerned from studies involving groups of individuals with probable food allergies. In the U.S., eggs and peanuts are also common allergenic foods for infants, along with soybeans, tree nuts, fish, and wheat (Bock and Atkins, 1990; Burks et al., 1988; Sampson and McCaskill, 1985). Among adults in the U.S., peanuts are probably the most common allergenic food (Taylor et al., 1999). Seafood allergies, especially to crustaceans (shrimp, crab, lobster), are also rather common among adults (Lehrer et al., 1992). Fewer studies have been conducted on the prevalence of specific types of food allergies in adults in the U.S. or other countries. The prevalence of specific types of food allergies may vary among population groups based upon their eating habits (Taylor et al., 1999). Peanut allergy appears to be more common in North America than in other parts of the world. This observation may relate to the popularity of peanut butter in North America. As other countries, such as the United Kingdom, have adopted the North American affection for peanuts and peanut products, the prevalence of peanut allergy in those countries appears to be rising (Emmett et al., 1999). Another example would be buckwheat allergy. Buckwheat allergy appears to be rather common among adults in certain southeast Asian countries such as Japan and South Korea (Kang and Min, 1984). In contrast, buckwheat allergy would be rather uncommon in the U.S. The difference is likely due to the popularity of buckwheat noodles in the cuisine of certain southeast Asian countries. Such observations have profound implications for product developers. If a highly successful buckwheat product was introduced into the U.S. or if buckwheat noodles became popular in American Asian cuisine, the prevalence of buckwheat allergy would likely increase.
--- PAGE BREAK ---
The Big Eight. Eight foods or food groups are thought to account for more than 90% of all IgE-mediated food allergies on a worldwide basis (Bousquet et al., 1998; FAO, 1995). These foods or food groups are milk, eggs, fish (all species of finfish), crustacea (shrimp, crab, lobster, crayfish), peanuts, soybeans, tree nuts (almonds, walnuts, pecans, cashews, Brazil nuts, pistachios, hazelnuts also known as filberts, pine nuts also known as pinyon nuts, macadamia nuts, chestnuts, and hickory nuts), and wheat. In 1995, an expert consultation of the Food and Agriculture Organization of the United Nations determined that these eight foods or food groups were the most common causes of food allergy on a worldwide basis (FAO, 1995). Subsequently this list was adopted by the Codex Alimentarius Commission in 1999 (CAC, 1999). These foods and food groups have come to be known as the “Big Eight” (Table 2). More than 160 other foods have been documented as causing food allergies less frequently (Hefle et al., 1996).
Basically, any food that has protein has the potential to elicit an allergic reaction among susceptible individuals. Beyond the Big Eight, in certain geographic regions other foods or food groups may frequently cause IgE-mediated food allergies. Celery allergy, for example, is rather common in some European countries (Wuthrich et al., 1990). The prevalence of buckwheat allergy in southeast Asia has already been mentioned. And, sesame seed allergy is very common in middle Eastern countries and countries where the ethnic population of middle Easterners is high; this may be due to the popularity of tahini, a paste made from sesame seeds, in the diets (Kanny et al., 1996). Several countries including Canada have decided to add sesame seeds to the list of commonly allergenic foods for that country. A few other foods are worthy of mention because, although they less frequently cause allergies, they have been associated with severe reactions. These foods include molluscan shellfish (clams, oysters, etc.), sesame seeds, poppy seeds, sunflower seeds, cottonseed, and certain other legumes beyond peanuts and soybeans (the various types of dry beans, peas, lentils, and garbanzo beans also known as chick peas) (Atkins et al., 1988; Gall et al., 1990; Kagi and Wutrich, 1993; Kalyoncu and Stalenheim, 1993; Kanny et al., 1996; Maeda et al., 1991; Martin et al., 1992; Noyes et al., 1979). However, a rather large percentage of the 160 or more other allergenic foods has been reported to elicit severe allergic reactions in isolated cases (Hefle et al., 1996).
Food Allergens. The allergens in foods are almost always naturally occurring proteins. Foods contain millions of individual proteins, but only a comparative few of the proteins have been documented to be allergens (Bush and Hefle, 1996; Taylor, 1996). Some foods such as milk, eggs, and peanuts are known to contain multiple allergenic proteins (Bush and Hefle, 1996). Other foods such as Brazil nuts, shrimp, and codfish contain only one major allergenic protein (Bush and Hefle, 1996). However, the majority of the proteins, even those from commonly allergenic foods, are incapable of eliciting IgE production. Although the common allergenic foods listed above tend to be good sources of protein, other common protein-rich foods such as beef, pork, chicken, and turkey are rarely allergenic. No common structural features have allowed distinctions to be made between those proteins that are capable of eliciting IgE production and those that are not. Allergenic proteins, however, tend to be major proteins in the implicated foods, resistant to digestion, and stable to processing operations, particularly heat processing (Taylor and Lehrer, 1996).
Development of IgE-Mediated Food Allergies. Genetics play an important role in the development of IgE-mediated allergies of all types, including food allergies (Kjellman and Bjorksten, 1997). Allergies are much more likely to develop in children born to parents who have allergies (either to food, pharmaceutical, or environmental allergens) than amongst children born to parents with no history of allergies (Taylor et al., 1999). However, the nature of the allergy that develops is not genetically controlled. Therefore, the children of pollen-allergic parents are at increased risk for development of food allergies as are the children of food-allergic parents. The risk is greater if both parents have allergies than it is if only one parent has allergies.
Infants are the most likely to develop food allergies (Sampson, 1990; Taylor, 1987). However, sensitization to foods can occur at any age. Infants do not appear to develop allergies in utero (Kjellman and Bjorksten, 1997), but can be sensitized during the first few days of life. Obviously, the newborn infant encounters through the first few years of life dozens of new foods and probably hundreds of thousands of food proteins that have antigenic and possibly allergenic potential. For most of these foods and their proteins, infants develop oral tolerance (Strobel, 1997). Infants appear to be at increased risk for the development of IgE-mediated food allergies in part because their digestive processes may not be fully developed but primarily because they have not yet had the opportunity to develop oral tolerance. Certain foodborne proteins seem to be much more likely than others to cause allergic sensitization (Bush and Hefle, 1996). The factors involved in sensitization and the development of IgE-mediated food allergies are not yet fully understood.
--- PAGE BREAK ---
Prevention of IgE-Mediated Food Allergies. The prevention of the development of IgE-mediated food allergies among high-risk infants (those born to parents with histories of allergies) has been a long-sought goal. However, the results of several large clinical trials of high-risk infants followed for several years suggest that the development of IgE-mediated food allergies can be delayed but not prevented (Zeiger and Heller, 1995). The maternal diet during pregnancy does not seem to be a factor (Zeiger and Heller, 1995), because sensitization does not occur in utero. The avoidance of commonly allergenic foods such as cows’ milk, eggs, and peanuts in the infant diet during the first few years of life often delays the development of food allergies, but food allergies may still develop after solid foods are introduced (Hattevig et al., 1989; Zeiger and Heller, 1995). Avoidance can be accomplished through breast-feeding or the feeding of hypoallergenic infant formula (Kjellman and Bjorksten, 1997; Zeiger and Heller, 1995). Many pediatricians recommend breast-feeding for infants born to parents with histories of IgE-mediated allergies. Lactating women can apparently transmit potentially sensitizing levels of food allergens through their milk to nursing infants (Van Asperen et al., 1983). Apparently, intact or partially intact allergenic proteins are able to survive maternal digestive processes and be absorbed via the lymph and transferred immunologically intact into breast milk (Van Asperen et al., 1983). Infants have been sensitized through breast-feeding to peanuts, cows’ milk, and eggs (Van Asperen et al., 1983). The avoidance of peanuts in the maternal diet during the lactation period is often advocated as a preventive measure. However, the exclusion of milk and eggs from the maternal diet during lactation is not usually recommended due to their nutritional importance coupled with the low likelihood of allergic sensitization through breast-milk (Taylor et al., 1999). The use of hypoallergenic infant formula to prevent or delay the development of IgE-mediated food allergies is less commonly practiced. However, the use of partial whey hydrolysate formulae for this purpose may show some promise (Vandenplas et al., 1992), although such partial hydrolysates are not safe for consumption by infants who are already sensitized to cows’ milk (Businco et al., 1989; Ellis et al., 1991). Apparently, the partial hydrolysis of the whey proteins increases the likelihood of the development of oral tolerance as opposed to allergic sensitization.
Natural History of IgE-Mediated Food Allergies. Most food allergies developed in infancy are outgrown in infancy or early childhood. Many infants outgrow their food allergies, often within a matter of a few months (Bock, 1982; Hill and Hosking, 1992). Allergies to certain foods such as cows’ milk, eggs, and soybeans are much more likely to be outgrown than allergies to other foods such as peanuts (Bock, 1982; Bock and Atkins, 1989). Peanut allergy is almost never outgrown. The loss of allergic sensitivity to a particular food probably results from the development of immunological, oral tolerance (Taylor et al., 1986a). However, the basis for the differences between specific foods (e.g., milk vs. peanuts) in the development of oral tolerance is not understood.
Cross-Reactions between Related Allergenic Foods. Allergic consumers sometimes experience cross-reactions between closely related foods. For example, with the crustacea, most sensitive individuals are allergic to all of the various species of shrimp, crab, lobster, and crayfish (Lehrer, 1986). However, these individuals can often eat other seafoods including finfish and molluscan shellfish. Similar cross-reactivity also exists between cows’ milk and goats’ milk (Bellioni-Businco et al., 1999) and between eggs of various avian species (Langeland, 1983). With other food groups, the situation can be quite complicated. For example, peanuts are the most commonly allergenic legumes. Most peanut-allergic individuals can eat other legumes without incident (Bernhisel- Broadbent and Sampson, 1989). A few peanut-allergic individuals are also allergic to soybeans (Herian et al., 1990), although this may not necessarily represent true cross-reactivity. With fish, individuals often experience reactions to more than one species of fish, but some fish are tolerated by some fish-allergic individuals and no definite patterns of reactivity have been identified (Bernhisel-Broadbent et al., 1992; de Martino et al., 1990).
Cross-Reactions between Food and Environmental Allergens. Cross-reactions are frequently observed between pollens and certain foods, especially fruits and vegetables (Ballmer-Weber et al., 2000; Calkoven et al., 1987; van Ree and Aalberse, 1993; Wuthrich et al., 1990). This is the oral allergy syndrome, which typically involves mild reactions as previously mentioned. Examples include cross-reactions between birch pollen and apples, ragweed pollen and melons, and mugwort pollen and celery (Ballmer-Weber et al., 2000; Calkoven et al., 1987; Enberg et al., 1987; van Ree and Alberse, 1993; Wuthrich et al., 1990). Cross-reactions have also been noted between latex allergies, a common problem among health-care workers, and certain foods including bananas, kiwis, avocados, and chestnuts (Blanco et al., 1994).
Effect of Food Processing on Allergens. As mentioned earlier, the allergens in foods are typically proteins that are stable to heat processing (Taylor and Lehrer, 1996). So, heat-processed forms of commonly allergenic foods often retain their allergenicity (Herian et al., 1993; Nordlee et al., 1981). The only exception would be the pollen-related allergens found in fresh fruits and vegetables and involved in oral allergy syndrome; these allergens are usually destroyed by heat processing (Dreborg and Foucard, 1983), although the necessary extent of the heat process has not been well documented in most cases. For the most common allergenic foods, all forms of those foods should be considered allergenic unless proven otherwise. Testing has indicated that most forms of peanuts and soybeans, for example, retain their allergenicity (Herian et al., 1993; Nordlee et al., 1981). Other processing techniques have not been so well investigated for their effects on the allergenicity of the resulting products. Since allergens are resistant to proteolysis, fermentation usually fails to eliminate allergenicity (Taylor and Lehrer, 1996). For example, although fermented soybean products are reduced in allergenicity, some allergenic activity is retained (Herian et al., 1993).
--- PAGE BREAK ---
If the protein fraction is removed during processing, however, the resulting product or ingredient might be safe because the allergen has been removed. The classic example is the processing of edible oils from peanuts and soybeans (Bush et al., 1985; Hourihane et al., 1997a; Taylor et al., 1981). Clinical challenge trials in peanut- and soybean-allergic individuals have documented that highly refined peanut and soybean oil are safe for individuals with allergies to the source material (Bush et al., 1985; Hourihane et al., 1997a; Taylor et al., 1981).
Other ingredients may be derived from allergenic sources. Examples include certain flavoring formulations, starch, lecithin, and gelatin. Flavors can occasionally contain protein residues from allergenic foods (Taylor and Dormedy, 1998). Starch is often made from corn or some other source that is rarely allergenic. Occasionally, starch is made from wheat, although the level of protein residues is quite low and adverse reactions to wheat starch have not been reported. Lecithin can be made from either soybean or egg and can contain allergenic residues (Muller et al., 1998). However, the degree of risk posed by the low residual levels of soybean allergens in lecithin remains unknown. Gelatin is most commonly made from beef and pork, foods that are rarely allergenic (Sakaguchi et al., 1996). However, gelatin can also be made from fish skins. The allergenicity of fish gelatin remains unknown (Sakaguchi et al., 1999).
Treatment. Allergic reactions to foods can be treated with certain drugs (Furukawa, 1988; Simons, 1998). Antihistamines can counteract the effects of histamine (Simons, 1998), although these drugs do not counteract the effects of the other mediators released from basophils and mast cells. Epinephrine (adrenaline) is considered the life-saving drug for individuals at risk of severe anaphylactic shock-type reactions to foods (Sampson et al., 1992). Epinephrine is available in self-injectable form. Consumers with a history of severe anaphylactic reactions to foods should have a prescription for epinephrine and carry the medication at all times. To be most effective, epinephrine must be administered early in the course of the allergic reaction. However, an examination of severe food-allergic reactions resulting in deaths or near-deaths reveals a delay in the administration of epinephrine as a key contributing factor to the severe outcome (Sampson et al., 1992).
The specific avoidance diet is the only prophylactic approach to the treatment of food allergies (Taylor et al., 1986a; Taylor et al., 1999). For example, individuals allergic to peanuts must simply avoid ingesting peanuts. The construction of safe and effective avoidance diets is often a challenge for individuals with food allergies. With packaged foods, these individuals must spend considerable time in the scrutiny of ingredient declarations on product labels. They must be taught to recognize the many terms that may signify the presence of food components or ingredients derived from their offending food(s). Some foods, especially in foodservice settings, are sold without ingredient statements. Clearly, the allergic consumer can encounter many hazardous situations in such circumstances and must be trained to be extremely vigilant. Also, exposure to very small amounts of the offending food may be sufficient to elicit allergic reactions in some sensitive individuals, further complicating the necessary vigilance in the implementation of effective avoidance diets.
Threshold Doses for Allergenic Foods. As noted, individuals with IgE-mediated food allergies will experience symptoms on exposure to small amounts of the offending food. The interaction of a small amount of the allergen with specific IgE antibodies on the surface of the mast cell or basophil membrane triggers the release of massive quantities of mediators, which accounts for the low degree of threshold. The precise threshold doses for allergenic foods have not been carefully investigated and are likely to be variable from one allergic individual to another. In a recent study, Hourihane et al. (1997b) demonstrated that a group of individuals with peanut allergy displayed different thresholds for peanuts. The most sensitive individual among 12 tested subjects experienced an objective reaction when exposed to 2 mg of peanut protein. Other patients in the group did not even respond to the highest dose used in the challenge trial which was 50 mg of peanut protein. While this experiment clearly demonstrates that the threshold level is not zero, the threshold dose is quite low. Whether other allergenic foods have thresholds as low as those for peanuts remains to be determined.
Allergenicity of Foods Produced Through Agricultural Biotechnology. In the genetic modification of foods, genes are transferred from one organism to another. Because these genes code for the expression of a particular protein, novel proteins are expressed in the transgenic variety as a result. Because all allergens are proteins, the theoretical possibility exists that these novel proteins might be allergenic or that they might become allergenic. However, only a few of the many proteins found in nature are allergenic, so the probability of the transfer of an allergen is rather low. The potential allergenicity of the novel proteins expressed in the new varieties produced through agricultural biotechnology should be assessed in every case.
--- PAGE BREAK ---
The potential allergenicity of the novel proteins in transgenic varieties can be assessed (FAO/WHO, 2001; Metcalfe et al., 1996; Taylor and Hefle, 2001a). If a gene is transferred from a known allergenic source, the potential allergenicity of the expressed novel protein can be assessed with reasonable certainty by evaluating its reactivity by specific serum screening with serum containing IgE antibodies from individuals with well documented allergies to the source material. This assessment approach was demonstrated to be effective when it was determined that a Brazil nut protein transferred into soybeans to correct the inherent methionine deficiency of soybeans was the heretofore unidentified major allergen from Brazil nuts (Nordlee et al., 1996). The company involved abandoned further commercial interest in these transgenic soybeans as a result. It should be emphasized that genes are not often obtained from known allergenic sources in the development of commercial transgenic varieties. However, when genes are transferred from known allergenic sources, it must be assumed that the gene encodes for an allergenic protein unless proven otherwise.
More typically, genes are obtained from sources with no history of allergenicity. In these situations, a decision-tree approach is advocated for the assessment of the potential allergenicity of the novel protein. While no single test can perfectly predict the potential allergenicity of a particular novel protein from a source with no history of allergenicity, the application of a series of tests provides reasonable assurance that the novel protein is not likely to become an allergen. Several decision-tree approaches have been developed (FAO/WHO, 2001; Metcalfe et al., 1996; Taylor and Hefle, 2001a). These approaches rely upon evaluation of the source of the gene and its history of allergenicity, the sequence homology of the novel protein to known allergens, the immunoreactivity of the novel protein with serum IgE from individuals with known allergies to the source of the transferred gene, the immunoreactivity of the serum IgE from individuals with known allergies to sources that are broadly related to the source of the novel gene (e.g., grass pollen allergic individuals in cases where the gene is obtained from monocot sources), the pepsin resistance or digestive stability of the novel protein, and the immunogenicity of the novel protein in validated animal models (Fig. 3). Other factors especially the level of expression of the novel protein in the food are also likely to be important since allergies are usually elicited by food proteins where dietary exposure is comparatively high.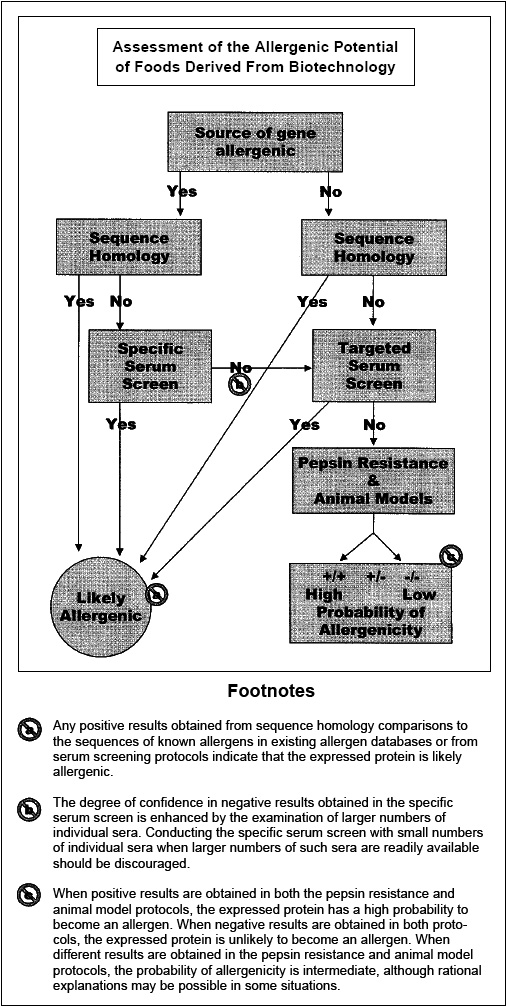
Foods produced through agricultural biotechnology including corn, soybeans, canola, and potatoes are already reaching the consumer marketplace. The potential allergenicity of the novel proteins expressed in these commercial products has been assessed using the approaches indicated above. The novel proteins in these approved transgenic varieties are not obtained from known allergenic sources, are not structurally homologous to known food or environmental allergens, and are sensitive to digestive proteolysis. Furthermore, the novel proteins are expressed at very low levels in the edible portions of these modified crops. Thus, the likelihood of allergenicity from this current generation of crops produced by agricultural biotechnology is virtually nil.
Considerable publicity has surrounded one particular transgenic variety, the so-called StarLink™ corn. StarLink corn was never approved for human use, but was unfortunately approved for animal feed use. When segregation failed, some StarLink corn residues were found in corn-based food products intended for human consumption. Although the gene inserted in StarLink corn was obtained from a source with no history of allergenicity and the novel protein was not structurally similar to known food or environmental allergens, the novel protein was comparatively more resistant to digestive proteolysis than other novel proteins that had been approved for other genetically modified crops. For this reason, StarLink corn was never approved for human consumption. However, given the very low level of exposure to the novel protein in StarLink corn in corn-based food products, the likelihood of allergic sensitization to this particular novel protein is low. Despite that and due to the concerns about the potential allergenicity of this transgenic variety, it has been withdrawn from the marketplace, although traces may remain for one or more growing seasons. This episode emphasizes the importance of assessing the potential allergenicity of transgenic varieties as part of the overall safety evaluation process.
Agricultural biotechnology can also be used to decrease the inherent allergenicity of foods. The proteins in specific foods that are responsible for allergic reactions could be removed or altered through agricultural biotechnology. While no commercial examples exist yet of the application of this possibility, it is an active area of research with peanuts.
--- PAGE BREAK ---
Non-IgE Cell-Mediated Reactions
As noted earlier, cell-mediated allergic reactions, also known as delayed hypersensitivity reactions, have an onset time of 6–24 hours after ingestion of the offending food. The reactions develop slowly, reaching a peak at approximately 48 hours and then slowly subsiding over 72–96 hours. Cell-mediated food allergies involve the interaction between specific antigens or allergens from the food and sensitized, tissue-bound T cells that release inflammatory mediators when sensitized (Sampson, 1991). The role of cell-mediated reactions in food allergies remains somewhat uncertain. Compelling and mounting evidence has accumulated, however, indicating that celiac disease occurs through a T cell-mediated mechanism (Ferguson, 1997; Strober, 1986).
Celiac Disease. Also known as celiac sprue or gluten-sensitive enteropathy, celiac disease is a malabsorption syndrome occurring in sensitive individuals upon the consumption of wheat, rye, barley, triticale, spelt, and kamut (Ferguson, 1997; Lemke and Taylor, 1994 ). The role of oats in celiac disease has recently been questioned. Apparently, oats and oat products that are totally free of wheat, rye, and barley, are safe for celiac sufferers to consume (Janatuinen et al., 1995). After consumption of the offending grains or products made from these grains, the absorptive epithelial cells in the small intestine are damaged by an inflammatory process (Ferguson, 1997). As a result, absorption of nutrients through the epithelium is compromised. The loss of absorptive function along with the ongoing inflammatory process results in a severe malabsorption syndrome characterized by diarrhea, bloating, weight loss, anemia, bone pain, chronic fatigue, weakness, muscle cramps, and, in children, failure to gain weight and growth retardation (Lemke and Taylor, 1994; Skerritt et al., 1990). A fraction of wheat, the gliadin fraction, and related fraction in barley and rye are associated with initiation of celiac disease in susceptible individuals (Skerritt et al., 1990).
Celiac disease is an inherited trait, but the inheritance is complex and poorly understood. Celiac disease occurs in about 1 of every 3000 individuals in the U.S. (Kasarda, 1978). In some other parts of the world, celiac disease occurs more frequently. The highest prevalence occurs among individuals in certain regions of Europe (Greco et al., 1992; Kasarda, 1978). Celiac disease seems to occur more frequently among Europeans than among Americans of European descent for unexplained reasons. Celiac disease rarely occurs among individuals of Chinese or African descent (Ferguson, 1997).
The treatment of celiac disease involves the avoidance of wheat, rye, barley, triticale, spelt, kamut, and oats and products of these grains (Hartsook, 1984). The threshold dose of gliadin and related protein fractions needed to provoke celiac disease in sensitive individuals is not precisely known, but symptoms can be elicited by ingestion of small amounts of these grains (Lemke and Taylor, 1994). If a gluten-free diet is followed, the symptoms of celiac disease will resolve and the absorptive function of the small intestine will be restored. Most celiac sufferers adhere to very strict gluten-free diets. In the absence of information on the safety of products made from the offending grains, affected individuals often choose to avoid products that contain remarkably small amounts of protein from these sources including rye alcohol, wheat starch, malt extract, and vinegar. The wisdom of such severely restricted avoidance diets remains to be established.
Food Intolerances
In contrast to true food allergies, food intolerances occur through non-immunological mechanisms. However, like true food allergies, food intolerances affect some individuals in the population but not all. Individuals suffering from food intolerances can usually tolerate small amounts of the offending food or food ingredient in their diet without ill effects. Food intolerances can be divided into three categories: anaphylactoid reactions, metabolic food disorders, and idiosyncratic illnesses.
Anaphylactoid Reactions. In IgE-mediated food allergies, the release of histamine and other mediators from the mast cells and basophils is mediated by the interaction of IgE with proteinaceous allergens, as described earlier. In contrast, anaphylactoid reactions are caused by substances that bring about the release of the same mediators from mast cells without the involvement of IgE (Lemke and Taylor, 1994). Some substance in the implicated food is presumed to destabilize the mast cell membranes allowing for the spontaneous release of histamine and the other mediators. However, no such histamine-releasing substance has ever been isolated or identified in foods, although this mechanism is well established with certain drugs. Strawberry sensitivity is usually cited as the best example of an anaphylactoid reaction. Although strawberries are known to cause adverse reactions (frequently urticaria) in susceptible individuals, there is little evidence for the existence of an IgE-mediated mechanism. Strawberries contain little protein, and no evidence has been found for the existence of a strawberry allergen. Furthermore, there is no evidence for the existence of strawberry-specific IgE in the sera of strawberry-sensitive individuals. Spontaneous histamine release is thus a plausible mechanism. However, if a substance exists in strawberries that destabilizes mast cell membranes, that substance has yet to be identified. The possibility that strawberry sensitivity is a form of oral allergy syndrome has not yet been excluded, and is an equally plausible mechanism.
--- PAGE BREAK ---
Metabolic Food Disorders. Metabolic food disorders result either from inherited defects in the ability to metabolize some component of food or from a genetically determined, enhanced sensitivity to some foodborne chemical that occurs through an altered metabolic pattern (Lemke and Taylor, 1994). Lactose intolerance is an example of an illness that occurs when a genetic deficiency affects the host’s ability to metabolize a food component (Kocian, 1988). In lactose intolerance, an inherited deficiency occurs in the amount of the enzyme, β-galactosidase, leading to an impaired ability to digest lactose. Favism is an example of a genetic deficiency that enhances the sensitivity to a foodborne chemical. In favism, a genetic deficiency in glucose-6-phosphate dehydrogenase in the erythrocyte results in an enhanced sensitivity to several hemolytic substances that occur naturally in fava beans (Mager et al., 1980). These two metabolic food disorders are the most common and best understood illnesses in this category of food intolerances.
Lactose Intolerance. Lactose is a dissaccharide and the principal sugar in milk. Normally, lactose is hydrolyzed into its constituent monosaccharides, galactose and glucose, in the small intestinal mucosa. These monosaccharides can then be absorbed and used as metabolic sources of energy. In lactose intolerance, the activity levels of β-galactosidase, the key hydrolytic enzyme that exists in the mucosal membranes of the small intestine, are diminished (Houts, 1988; Suarez and Savaiano, 1997). Since lactose cannot be absorbed in the small intestine unless it is hydrolyzed to glucose and galactose, the undigested lactose passes into the colon where it encounters large populations of bacteria. The colonic bacteria metabolize the lactose to CO2, H2, and H2O (Lemke and Taylor, 1994). Abdominal cramping, flatulence, and frothy diarrhea are the predominant symptoms of lactose intolerance (Bayless et al., 1975) and are the direct result of the action of the colonic bacteria on lactose. The symptoms vary in intensity in concert with the individual level of activity of β-galactosidase in the small intestine and the amount of lactose ingested.
Lactose intolerance is a fairly common metabolic food disorder. Lactose intolerance is especially prevalent among some ethnic groups in the world including Greeks, Arabs, Jews, black Americans, Hispanics, Japanese, and other Asians (Houts, 1988; Suarez and Savaiano, 1997). Only about 6-12% of Caucasians are affected (Suarez and Savaiano, 1997). Lactose intolerance can have its onset at any age, occurring as early as the age of three (Simoons, 1980). However, lactose intolerance tends to worsen with advancing age and is often more common and more severe among the elderly (Houts, 1988; Simoons, 1980). The level of intestinal b-galactosidase is usually sufficient at birth to allow digestion of lactose in mother’s milk, but susceptible individuals suffer as a result of decreased activity of this enzyme as life progresses. Lactose intolerance may also occur on a more transitory basis on occasion, secondary to another intestinal illness or infection such as a bout of viral gastroenteritis (Metcalfe, 1984b). Secondary lactose intolerance tends to subside rather quickly after the original illness is resolved.
Individuals with lactose intolerance are able to control their symptoms through the avoidance of dairy products containing lactose (Lemke and Taylor, 1994). However, many lactose-intolerant individuals can tolerate some lactose in their diets. The degree of tolerance for lactose is individualistic and variable among such individuals. Yogurt, sour cream, and acidophilus milk that contain active cultures of bacteria with b-galactosidase activity, are better tolerated by lactose-intolerant individuals than other dairy products (Kolars et al., 1984). Lactose-hydrolyzed milk is also available in the marketplace (Paige et al., 1975). And, lactose-intolerant individuals can add b-galactosidase to milk just before consumption, and this seems to be an effective practice (Barillas and Solomons, 1987). Certainly, the level of tolerance for dairy products is much higher with lactose intolerance than it is with IgE-mediated cows’ milk allergy (Taylor, 1990).
Favism. Individuals with an inherited deficiency of the enzyme, glucose-6- phosphate dehydrogenase (G6PDH), in their erythrocytes are susceptible to favism. Symptoms occur after consumption of fava beans or the inhalation of pollen from the Vicia faba plant (Mager et al., 1980). Fava beans contain vicine and convicine, naturally occurring oxidants that are able to damage the erythrocyte membranes of G6PDH-deficient individuals causing hemolysis and the symptoms of hemolytic anemia (Marquardt, 1989). G6PDH is a critical enzyme in erythrocytes because it helps to maintain adequate levels of the reduced form of glutathione (GSH) and nicotinamide adenine dinucleotide phosphate (NADPH). GSH and NADPH help to avert oxidative damage to erythrocytes. In individuals who are G6PDH-deficient, this protective mechanism is nonfunctional and the oxidants in fava beans can exert their hemolytic effects. In rare cases with repeated exposure, more severe symptoms can occur including hemoglobinuria, jaundice, and renal failure. The onset time after ingestion of the fava beans ranges from 5 to 24 hours. The illness is self-limited, however, with symptoms resolving promptly and spontaneously upon avoidance of further exposure.
G6PDH deficiency is very common and affects approximately 100 million individuals on a worldwide basis (Mager et al., 1980). The prevalence is highest among Oriental Jewish groups in Israel, Sardinians, Cypriot Greeks, African Americans, and certain African populations. This inherited trait is virtually absent among Caucasians, North American Indians, and Eskimos. Despite the high prevalence of G6PDH deficiency, favism is an uncommon occurrence because fava beans are not frequently eaten except in Mediterranean and Middle Eastern locales.
Idiosyncratic Illnesses
Some individualistic adverse reactions to foods are idiosyncratic in that the mechanism for these illnesses is unknown. Many reports, mostly anecdotal, have occurred regarding illnesses in certain individuals attributed to certain specific foods or food ingredients. Conceivably, a large number of different mechanisms could be involved in these idiosyncratic reactions. The symptoms involved in idiosyncratic reactions range from trivial to severe, life-threatening reactions (Taylor et al., 1989b).
--- PAGE BREAK ---
The role of specific foods or food ingredients in the causation of these idiosyncratic reactions remains to be determined in many cases. The cause-and-effect relationships can only be established through carefully controlled DBPCFCs (Taylor et al., 1989b). A positive DBPCFC would definitely confirm that the specific food or food ingredient is involved in the particular adverse reaction. The mechanism of the adverse reaction, however, cannot be determined from the positive DBPCFC alone. A negative DBPCFC indicates either that foods are not involved in causation of the reaction or at least that the specific food or food ingredient used in the challenge was wrongly incriminated. Unfortunately, DBPCFCs are rarely performed to establish convincingly that a specific food or food ingredient is associated with a particular idiosyncratic reaction.
The role of specific foods or food ingredients are firmly established in a few of the many alleged foodborne idiosyncratic reactions. Sulfite-induced asthma is perhaps the best example (Bush and Taylor, 1998). In the case of sulfite-induced asthma, many clinicians have documented the role of sulfites in the provocation of asthma in dozens of patients using DBPCFC protocols (Bush and Taylor, 1998). Aspartame has been identified as a causative factor in two subjects using DBPCFC (Kulczycki, 1986). However, other cases of aspartame-induced urticaria have not been identified so this may be a rather rare phenomenon.
For many other alleged idiosyncratic reactions to specific foods or food ingredients, the association with the specific food or food ingredient has not been conclusively documented through DBPCFCs. Examples would include the role of chocolate or aspartame in migraine headache; the roles of BHA, BHT, tartrazine, benzoates, or parabens in chronic urticaria; the role of tartrazine in asthma; the role of monosodium glutamate (MSG) in asthma or MSG Symptom Complex; and the role of sugar in aggressive behavior (Bush and Taylor, 1998). A thorough critique of the many studies that have been conducted on the role of these foods or food ingredients in the causation of these idiosyncratic reactions is beyond the scope of this particular review. However, very few of the clinical studies have used double-blind and placebo-controlled trial designs. Furthermore, many of the studies have involved individuals with chronic, episodic symptoms such as chronic urticaria or asthma and the clinical investigators have removed critical medications from the patients before initiating the challenge trials. In such cases, the observed symptoms might be due either to the challenge substance or to the withdrawal of medications that controlled the condition. With such critical clinical design flaws, the role of these specific foods and food ingredients in these particular idiosyncratic illnesses remains unproven. Furthermore, psychological disorders may be involved in perceived reactions to specific foods or food ingredients (King, 1984; Selner and Staudenmayer, 1997).
In a few cases, the role of specific foods or food ingredients in idiosyncratic reactions has been disproven by careful clinical investigations. However, consumers may persist in their belief that such reactions occur. The outstanding example of such a reaction is the role of artificial food colors in hyperkinetic behavior in children. Several decades ago, Dr. Benjamin Feingold implicated artificial food colorants as causative factors in hyperkinesis on the basis of poorly controlled trials and anecdotal experiences (Feingold, 1975). The resulting publicity on the Feingold hypothesis was considerable, and, consequently, many consumers became convinced of a relationship between ingestion of artificial food colorants and provocation of hyperkinetic behavior in children. Subsequently, several double-blind, placebo-controlled challenge trials have been conducted with artificial food colorants and have demonstrated convincingly that few, if any, hyperkinetic children are adversely affected by the ingestion of these food colorants (Harley et al., 1978a). Despite this evidence, many consumers persist in their belief regarding the role of artificial food colorants in hyperkinetic behavior.
A similar situation exists with respect to monosodium glutamate where the involvement of MSG intake in the so called Chinese Restaurant Syndrome, now more appropriately called MSG Symptom Complex, has been alleged so often that it is now accepted as fact by many consumers. However, the role of MSG in MSG Symptom Complex has not been corroborated in carefully controlled clinical challenge studies (Kenny, 1986; Tarasoff and Kelly, 1993). More recently, MSG intake has been linked to asthma (Allen et al., 1987). However, the role of MSG in provocation of asthma seems questionable at best when patients are evaluated using a DBPCFC protocol (Bush and Taylor, 1998). The alleged role of tartrazine, also known as FD&C Yellow #5, in asthma and chronic urticaria is also very questionable in the light of DBPCFCs (Bush and Taylor, 1998; Stevenson et al., 1986). Yet, undeclared tartrazine remains the basis for a large number of FDA recalls.
Sulfite-Induced Asthma. Sulfiting agents allowed for use in foods include sulfur dioxide, potassium metabisulfite, sodium metabisulfite, potassium bisulfite, sodium bisulfite, and sodium sulfite. Sulfiting agents have been used as food ingredients for many years, because they have many important technological benefits (Taylor et al., 1986b). Sulfites also occur naturally in some foods, especially fermented foods (Taylor et al., 1986b). However, residual levels of sulfites in foods vary from a few ppm arising mostly from natural sources, to less than 10 ppm to >1,000 ppm as a result of additive usage.
--- PAGE BREAK ---
Although sulfites have been used for centuries, they have been implicated as triggers of asthma in some sensitive individuals only in recent years (Bush and Taylor, 1998; Stevenson and Simon, 1981). The reactions usually occur within a few minutes after the ingestion of a provoking dose of sulfite. The reactions can be quite severe on occasion and deaths have been attributed to sulfite-induced asthma (Bush and Taylor, 1998). The role of sulfites in the causation of asthma in susceptible individuals has been well documented by DBPCFC (Bush and Taylor, 1998; Stevenson and Simon, 1981). Other symptoms have also been alleged to occur as a result of sulfite sensitivity but these reports have been largely anecdotal and unverified by DBPCFC (Bush and Taylor, 1998).
Sulfite-induced asthma affects only a small percentage of all asthmatic individuals (Bush et al., 1986). The prevalence among severe asthmatics who are dependent upon steroid-based drugs for control of their symptoms appears to be in the range of 4–7%, while mild asthmatics do not seem to be very susceptible to sulfite ingestion (Bush et al., 1986). Thus, the overall prevalence of sulfite-induced asthma is estimated at 1.0–1.5% of the total asthmatic population (Bush et al., 1986).
Sulfite-induced asthmatics display thresholds for sulfites (Bush and Taylor, 1998; Taylor et al., 1988). While the ingestion of high doses of sulfite in highly sulfited foods and beverages can be quite hazardous for susceptible individuals, the ingestion of sulfited foods with lower levels of residual sulfite (<100 ppm as total SO2) seems to present little risk (Taylor et al., 1988). Thus, sulfite-sensitive asthmatics must be alert to the presence of sulfites in foods at levels that are required to be declared on the ingredient statement, but are at no risk from ingestion of sulfites from foods that have levels of residual sulfite below the detection limit of current assay procedures (<10 ppm as total SO2).
The mechanism of sulfite-induced asthma is not known. Several different mechanisms have been proposed including IgE-mediated reactions, hypersensitivity to inhaled SO2 from ingestion of acidic foods and beverages, and sulfite oxidase deficiency (Bush and Taylor, 1998). However, none of these mechanisms has been proven, so sulfite-induced asthma remains an idiosyncratic illness.
Implications for Food Manufacturing
In accordance with the Food, Drug, and Cosmetic Act (Section 403(i)(2); 21 USC 343(i)(2); 21 CFR 101.4), food manufacturers are required to list all the ingredients of a food, with two exceptions (FDA/ORA, 2001). Section 403(i) of the FDC Act provides that spices, flavorings, and colorings may be declared collectively without naming each specific substance. FDA, however, has required on a substance-by-substance basis ingredients covered by the 403(i) exemption to be declared when necessary to protect individuals who experience adverse reactions to the substance, e.g., FD&C Yellow No. 5. The agency strongly encourages the declaration of an allergenic ingredient of a spice, flavor, or color, by either declaring the allergenic ingredient by its common or usual name in the ingredient list as a separate ingredient or parenthetically following the term spice, flavor, or color, or as a declaration attached at the end of the list of ingredients indicating the presence of a specific allergen (FDA/ORA, 2001). FDA regulations (21 CFR 101.100(a)(3)) exempt from ingredient declaration incidental additives, such as processing aids, present in food at insignificant levels and that do not have a technical or functional effect in the finished food. The FDA has stated, however, that an amount, although sometimes very small, of a substance that may cause an allergic reaction is not insignificant and therefore must be declared (FDA/CFSAN, 1996). The FDA is considering further its labeling requirements and whether any actions, e.g., clarification of labeling regulations or rulemaking, for labeling of allergenic ingredients is necessary (FDA/CFSAN, 1996; FDA/CFSAN/ONPLDS, 2001).
Further, manufacturers are required to follow Good Manufacturing Practices (GMPs). GMPs, described in a series of articles by Taylor and Hefle (2000a,b,c, 2001b), are a significant strategy for controlling food allergens and reducing the likelihood of allergic reactions. Key GMPs for preventing allergen cross-contamination via the use of shared equipment, often necessary for related food products with different formulations, are: dedication of lines or specific pieces of equipment, appropriate scheduling of manufacturing operations, and effective clean up between operations (Taylor and Hefle, 2000a). The effectiveness of these strategies can be assessed by sensitive, specific immunoassays, where available; otherwise, the standard must be visibly clean. GMPs for use of rework, sometimes an economic necessity, are maintaining a “like-into-like” policy; i.e., rework created from a specific formulation is only incorporated back into the same exact formulation and accurate labeling of the packaged product is maintained (Taylor and Hefle, 2000b).
Suppliers and co-packers must also have allergen control programs (Taylor and Hefle, 2000c). In working with their suppliers and co-packers, manufacturers must obtain accurate information on the allergen content of all product formulation substances, including composite ingredients such as flavors, spices, edible oils, and natural and artificial coloring. Manufacturers are also advised to regularly audit suppliers for effective allergen control practices, e.g., regarding sanitation and acquisition, storage, processing, and packaging of materials; and upon delivery to the manufacturers’ facilities, maintenance of the codes of all packages of ingredients and storage in a manner that will not contaminate other ingredients.
--- PAGE BREAK ---
Because ingestion of trace amounts of an allergen can provoke an allergic, potentially life-threatening reaction, GMPs are not necessarily always solely effective for sufficiently minimizing the risk of potential allergen contamination. In such cases, manufacturers use additional strategies for managing allergen control. These include addition of the allergic ingredient to the product as late in the manufacturing process as possible and scheduling of the processing operation of the most allergenic formulation just before the end of the shift prior to the major clean-up. Some food companies, with multiple facilities, have chosen to restrict the manufacturing of certain allergen-containing formulations, e.g., peanut-containing products, to dedicated facilities (Taylor and Hefle, 2000a).
A Food Allergy Issues Alliance, comprising food trade associations and other interested organizations, has been discussing options related to labeling of major food allergens and developed labeling guidelines. The guidelines address: identifying the major food allergens; using terms commonly understood by consumers; disclosing the presence of major food allergens when they are an intentional part of the food, regardless of source; and establishing guidelines for conditions when the use of supplemental statements is appropriate (NFPA, 2001a). Further, the National Food Processors Association developed for its members a Code of Practice to delineate general practices that can ensure effective strategies for food allergen management (NFPA, 2001b).
Conclusions
Taken together, food allergies and sensitivities are adverse reactions that affect a significant number of people in the overall population. The symptoms of any specific type of food allergy or food sensitivity are manifested only in a small segment of the total population. As a result of the individualistic nature of these illnesses, the public does not tend to view these illnesses as a major health concern. However, food allergies, and to a lesser extent, food intolerances are an increasingly important concern to food manufacturers. Consumers with various types of food allergies and intolerances must alter their lifestyles on a continuing basis to avoid the offending food or food ingredient. The industry must provide these consumers with the information necessary for them to practice such avoidance effectively. Ingredient labeling statements are the key to implementation of safe and effective avoidance diets. Manufacturers must also be aware that certain processing practices such as the use of shared equipment and the use of re-work can result in undeclared residues of allergenic foods existing in other products. These situations can be hazardous for allergic consumers especially if larger quantities of the allergenic foods are present on an undeclared basis. However, since individuals with food intolerances can usually tolerate some of the offending food in their diets, the practices in the processing environment are much less likely to result in the transfer of hazardous levels of the offending food or food ingredient.
INSTITUTE OF FOOD TECHNOLOGISTS
The Society for Food Science and Technology
221 N. LaSalle St., Ste. 300, Chicago, IL 60601–1291 USA
Tel: 312–782–8424 • Fax: 312–782–8348
E-mail: [email protected] • URL: http://www.ift.org
This and other Scientific Status Summaries are published by the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition in Food Technology. Scientific Status Summaries, which are not necessarily written by the Expert Panel, are rigorously peer-reviewed by the Expert Panel as well as by individuals outside the panel who have specific expertise in the subject. IFT’s Expert Panel on Food Safety and Nutrition, which studies significant food-related issues and oversees timely production of Scientific Status Summaries, comprises academicians representing expertise in one or more areas of food science/technology and nutrition.
The Scientific Status Summaries may be reprinted or photocopied without permission, provided that suitable credit is given.
Discover the value potential of the scientific insights contained within the Journal of Food Science. Wherever your interest lies in product or process development or improvement, you'll find important content in JFS. Peer-reviewed reports of original research and critical reviews of all basic and applied aspects of food science include:
Concise Reviews and Hypotheses in Food Science
Food Chemistry and Toxicology
Food Engineering and Physical Properties
Food Microbiology and Safety
Sensory and Nutritive Qualities of Food
Subscribe to JFS and get 10 issues per year—more than ever before—plus supplements on hot topics. Subscribers receive website access for online searches of the latest five years of JFS issues to aid their own research.
Order your subscription to JFS by calling 800-IFT-FOOD (800-438-3663) or 312-782-8424. Or visit www.ift.org to view rates and download a faxable order form.
"There is a message that science has to offer . . . the style of thinking, the integrity of argument; you honor your adversaries, and you put all the evidence on the table. You have an absolute obligation to feed your critics with all the data."
— Joshua Lederberg, 1958 Nobel Prize in Physiology and Medicine
The Nobel Prize Centennial 1901-2001
Information at www.nobel.se
The Journal of Food Science is a publication of the Institute of Food Technologists, a not-for-profit society for food science and technology.
Steve L. Taylor, Ph.D. and Susan L. Hefle, Ph.D.
The authors, both Professional Members of IFT, are co-directors, University of Nebraska Food Allergy Research & Resource Program, Food Processing Center, Lincoln, NE 68583-0919.
References
Adolphson, C.R., Gleich, G.J., Yunginger, J.W. 1986. Standardization of allergens. In “Manual of Clinical Immunology,” 3rd Ed., ed. N. Rose, H. Friedman, and J. Fahey, pp. 652-659, ASM Press, Washington, D.C.
Allen, D.H., Delohery, J., and Baker, G. 1987. Monosodium L-glutamate-induced asthma. J. Allergy Clin. Immunol. 80: 530-537.
Anderson, J.A. 1996. Allergic reactions to foods. Crit. Rev. Food Sci. Nutr. 36S: S19-S38.
Atkins, F.M., Wilson, M., and Bock, S.A. 1988. Cottonseed hypersensitivity: New concerns over an old problem. J. Allergy Clin. Immunol. 82: 242-250.
Ballmer-Weber, B.K., Vieths, S., Luttkopf, D., Heuschmann, P., and Wuthrich, B. 2000. Celery allergy confirmed by double-blind, placebo-controlled food challenge: A clinical study in 32 subjects with a history of adverse reactions to celery root. J. Allergy Clin. Immunol. 106(2): 373-378.
Barillas, C. and Solomons, N.W. 1987. Effective reduction of lactose maldigestion in preschool children by direct addition of beta-galactosidase to milk at mealtime. Pediatrics 79: 766-772.
Bayless, T.M., Rothfeld, B., Massa, C., Wise, L., Paige, D., and Bedine, M.S. 1975. Lactose and milk intolerance: Clinical implications. N. Engl. J. Med. 292: 1156-1159.
Beasley, R., Crane, J., Lai, C.K.W., and Pearce, N. 2000. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. 105: S466-S472.
Bellioni-Businco, B., Paganelli, R., Lucenti, P., Giampietro, P.G., Perborn, H., and Businco, L. 1999. Allergenicity of goat’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 103: 1191-1194.
Bernhisel-Broadbent, J. and Sampson, H.A. 1989. Cross-allergenicity in the legume botanical family in children with food hypersensitivity. J. Allergy Clin. Immunol. 83: 435-440.
Bernhisel-Broadbent, J., Scanlon, S.M., and Sampson, H.A. 1992. Fish hypersensitivity. I. In vitro results and oral challenge in fish allergic patients. J. Allergy Clin. Immunol. 89: 730-737.
Blackwell, B. and Marley, E. 1969. Monoamine oxidase inhibition and intolerance to foodstuffs. Bibl. Nutr. Diet. 11: 96-110.
Blanco, C., Carrillo, T., Castillo, R., Quiralte, J., and Cuevas, M. 1994. Latex allergy: Clinical features and cross-reactivity with fruits. Ann. Allergy 73: 309-314.
Bock, S.A. 1982. The natural history of food sensitivity. J. Allergy Clin. Immunol. 69: 173-177.
Bock, S.A. and Atkins, F.M. 1989. The natural history of peanut allergy. J. Allergy Clin. Immunol. 83: 900-904.
Bock, S.A. and Atkins, F.M. 1990. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J. Pediatr. 117: 561-567.
Bock, S.A., Buckley, J., Holst, A., and May, C.D. 1977. Proper use of skin tests with food extracts in diagnosis of hypersensitivity to food in children. Clin. Allergy 7: 375-383.
Bock, S.A., Lee, W.Y., Remigio, L., and May, C.D. 1978. Studies of hypersensitivity reactions to foods in infants and children. J. Allergy Clin. Immunol. 62: 327-334.
Bock, S.A., Sampson, H.A., Atkins, F.M., Zeiger, R.S., Lehrer, S., Sachs, M., Bush, R.K., and Metcalfe, D.D. 1988. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J. Allergy Clin. Immunol. 82: 986-997.
Bock, S.A., Muñoz-Furlong, A., and Sampson, H.A. 2001. Fatalities due to anaphylactic reactions. J. Allergy Clin. Immunol. 107: 191-193.
Bousquet, J. and Michel, F.B. 1988. Food allergy and asthma. Ann. Allergy 61: 70-74.
Bousquet, J., Bjorksten, B., Bruijnzeel-Koomen, C.A.F.M., Huggett, A., Ortolani, C., Warner, J.O., and Smith, M. 1998. Scientific criteria and selection of allergenic foods for labeling. Allergy 53(Suppl. 47): 3-21.
Burks, A.W., Mallory, S.B., Williams, L.W., and Shirrell, M.A. 1988. Atopic dermatitis: Clinical relevance of food hypersensitivity reactions. J. Pediatr. 113: 447-451.
Bush, R.K. and Hefle, S.L. 1996. Food allergens. Crit. Rev. Food Sci. Nutr. 36S: S119-S163.
Bush, R.K. and Taylor, S.L. 1998. Adverse reactions to food and drug additives. In “Allergy: Principles and Practice,” Vol. II, 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 1183-1198, Mosby, St. Louis, Mo.
Bush, R.K., Taylor, S.L., Nordlee, J.A., and Busse, W.W.1985. Soybean oil is not allergenic to soybean-sensitive individuals. J. Allergy Clin. Immunol. 76: 242-245.
Bush, R.K., Taylor, S.L., Holden, K., Nordlee, J.A., and Busse, W.W. 1986. Prevalence of sensitivity to sulfiting agents in asthmatic patients. Am. J. Med. 81: 816-820.
Businco, L., Cantani, A., Longhi, A., and Gampietro, G.P. 1989. Anaphylactic reactions to cow’s milk whey protein hydrolysate (Alfa-Re Nestle) in infants with cow’s milk allergy. Ann. Allergy 62: 333-335.
CAC. 1999. Report of the Twenty-third Session of the Codex Alimentarius Commission. Alinorm 99/37. Rome.
Calkoven, P.G., Aalbers, M., Koshte, V.L., Pos, O., Oei, H.D., and Aalberse, R.C. 1987. Cross-reactivity among birch pollen, vegetables and fruits as detected by IgE antibodies is due to at least three distinct cross-reactive structures. Allergy 42: 382-390.
Chiaramonte, L.T., Bassett, C.W., and Altman, D.R. 1999. Food allergy perception and reality. In “Food Hypersensitivity and Adverse Reactions: A Practical Guide for Diagnosis and Management,” ed. M. Frieri and B. Kettelhut, pp. 155-164, Marcel Dekker, N.Y.
Church, M.K., Holgate, S.T., Shute, J.K., Walls, A.E., and Sampson, A.P. 1998. Mast cell-derived mediators. In “Allergy: Principles and Practice, Vol. I,” 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 146-167, Mosby, St. Louis, Mo.
de Martino, M., Novembre, E., Galli, L., de Marco, A., Botarelli, P., Murano, E., and Vierucci, A. 1990. Allergy to different fish species in cod-allergic children: In vivo and in vitro studies. J. Allergy Clin. Immunol. 86: 909-914.
Dreborg, S. 1988. Food allergy in pollen-sensitive patients. Ann. Allergy 61: 41-46.
Dreborg, S. and Foucard, T. 1983. Allergy to apple, carrot and potato in children with birch pollen allergy. Allergy 38:167-172.
Ebner, C., Hirschwehr, R., Bauer, L., Breiteneder, H., Valenta, R., Ebner, H., Kraft, D. and Scheiner, O. 1995. Identification of allergens in fruits and vegetables: IgE cross reactivities with the important birch pollen allergens Bet v 1 and Bet v 2 (birch profilin). J. Allergy Clin. Immunol. 95: 962-969.
Ellis, M.H., Short, J.A., and Heiner, D.C. 1991. Anaphylaxis after ingestion of a recently introduced hydrolyzed whey protein formula. J. Pediatr. 118: 74-77.
Emmett, S.E., Angus, F.J., Fry, J.S., and Lee, P.N. 1999. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy 54: 380-385.
Enberg, R.N., Leickly, F.E., McCullough, J., Bailey, J., and Ownby, D.R. 1987. Watermelon and ragweed share allergens. J. Allergy Clin. Immunol. 79: 867-875.
Etkind, P., Wilson, M.E., Gallagher, K., and Cournoyer, J. 1987. Bluefish-associated scombroid poisoning. An example of the expanding spectrum of food poisoning from seafood. J. Am. Med. Assoc. 258: 3409-3410.
FAO. 1995. Food Allergies. Report of the Technical Consultation of the Food and Agriculture Organization of the United Nations, Rome. November 13-14.
FAO/WHO. 2001. Evaluation of Allergenicity of Genetically Modified Foods. Report of the Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology. Food and Agriculture Organization of the United Nations/World Health Organization. Rome. January 22-25.
FDA/CFSAN. 1996. Notice to manufacturers: Label declaration of allergenic substances in foods. June 10.
FDA/CFSAN/ONPLDS. 2001. “Dear colleague” letter on August 13, 2001 public meeting on the labeling of foods containing allergens. June, 29. Food and Drug Administration/Center for Food Safety and Applied Nutrition/Office of Nutritional Products, Labeling and Dietary Supplements, Washington, D.C.
FDA/ORA. 2001. Compliance Policy Guide: Compliance policy guidance for FDA staff. Sec. 555.250: Statement of policy for labeling and preventing cross-contact of common food allergens. Apr. 19. Food and Drug Administration/Office of Regulatory Affairs, Washington, D.C.
Feingold, B.F. 1975. “Why is Your Child Hyperactive?,” Random House, N.Y.
Ferguson, A. 1997. Gluten-sensitive enteropathy (celiac disease). In “Food Allergy: Adverse Reactions to Foods and Food Additives,” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon, pp. 287-301, Blackwell Scientific, Cambridge Mass.
Furukawa, C.T. 1988. Comparative trials including a beta 2 adrenergic agonist, a methylxanthine, and a mast cell stabilizer. Ann. Allergy 60: 472-476.
Gall, H., Forck, G., Kalveram, K.-J., and von Lersner-Lenders, S. 1990. Immediate-type allergy to legume foods. Allergologie 13: 352-355.
Greco, L., Maki, M., Di Donato, F., and Visakorpi, J.K. 1992. Epidemiology of coeliac disease in Europe and the Mediterranean area. In “Common Food Intolerances. 1: Epidemiology of Coeliac Disease,” ed. S. Auricchio and J.K. Visakorpi, pp. 25-44, Karger, Basel.
Gryboski, J.D. 1991. Gastrointestinal aspect of cow’s milk protein intolerance and allergy. Immunol. Allergy Clin. N. Am. 11: 773-797.
Harley, J.P., Matthews, C.G., and Eichman, P. 1978a. Synthetic food colors and hyperactivity in children: A double-blind challenge experiment. Pediatrics 62: 975-983.
Harley, J.P., Ray, R.S., Tomasi, L., Eichman, P.L., Matthews, C.G., Chun, R., Cleeland, C.S., and Traisman, E. 1978b. Hyperkinesis and food additives: Testing the Feingold hypothesis. Pediatrics 61: 818-828.
Hartsook, E.I. 1984. Celiac sprue: Sensitivity to gliadin. Cereal Foods World 29: 157-158.
Hattevig, G., Kjellman, B., Sigurs, N., Bjorksten, B., and Kjellman, N.I.M. 1989. Effect of maternal avoidance of eggs, cow’s milk and fish during lactation upon allergic manifestations in infants. Clin. Exp. Allergy 19: 27-32.
Hefle, S.L., Nordlee, J.A., and Taylor, S.L. 1996. Allergenic foods. Crit. Rev. Food Sci. Nutr. 36S: S69-S89.
Herian, A.M., Taylor, S.L., and Bush, R.K. 1990. Identification of soybean allergen by immunoblotting with sera from soy-allergic adults. Int. Arch. Allergy Appl. Immunol. 92: 193-198.
Herian, A.M., Taylor, S.L., and Bush, R.K. 1993. Allergenicity of various soybean products as determined by RAST inhibition. J. Food Sci. 58: 385-388.
Hill, D.J. and Hosking, C.S. 1992. Patterns of clinical disease associated with cow milk allergy in childhood. Nutr. Res. 12: 109-121.
Hill, D.J., Hosking, C.S., Zhie, C.Y., Leung, R., Baratwidjaja, K., Iikura, Y., Iyngkaran, N., Gonzalez-Andaya, A., Wah, L.B., and Hsieh, K.H. 1997. The frequency of food allergy in Australia and Asia. Environ. Toxicol. Pharmacol. 4:101-110.
Host, A. and Halken, S. 1990. A prospective study of cow’s milk allergy in Danish infants during the first three years of life. Allergy 45: 587-596.
Hourihane, J.O’B., Bedwani, S.J., Dean, T.P., and Warner, J.O. 1997a. Randomised, double-blind, crossover challenge study of allergenicity of peanut oils in subjects allergic to peanut. Br. Med. J. 314: 1084-1088.
Hourihane, J.O’B., Kilburn, S.A., Nordlee, J.A., Hefle, S.L., Taylor, S.L., and Warner, J.O. 1997b. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: A randomized, double-blind, placebo-controlled food challenge study. J. Allergy Clin. Immunol. 100: 596-600.
Houts, S.S. 1988. Lactose intolerance. Food Technol. 42: 110-113.
IFT. 1985. Food allergies and sensitivities. A Scientific Status Summary by the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition. Food Technol. 39(9): 65-71.
Jakobsson, I. and Lindberg, T. 1979. A prospective study of cow’s milk protein intolerance in Swedish infants. Acta Pediatr. Scand. 68: 853-859.
Janatuinen, E.K., Pikkarainen, P.H., Kemppainen, T.A., Kosma, V.M., Jarvinen, R.M., Uusitupa, M.I.J., and Julkunen, R.J.K. 1995. A comparison of diets with and without oats in adults with celiac disease. N. Engl. J. Med. 333: 1033-1037.
Kagi, M.K. and Wuthrich, B. 1993. Falafel burger anaphylaxis due to sesame seed allergy. Ann. Allergy 71: 127-129.
Kalyoncu, A.F. and Stalenheim, G. 1993. Allergy to poppy seed. Allergy 48: 295.
Kang, S.Y. and Min, K.U. 1984. Three cases of buckwheat allergy. J. Korean Med. Assoc. 27: 765-768.
Kanny, G., de Hauteclocque, C., and Moneret-Vautrin, D.A. 1996. Sesame seed and sesame seed oil contain masked allergens of growing importance. Allergy 51: 952-957.
Kaplan, A.P. 1998. Urticaria and angioedema. In “Allergy: Principles and Practice, Vol. II, 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 1104-1122, Mosby, St. Louis.
Kasarda, D.D. 1978. The relationship of wheat protein to celiac disease. Cereal Foods World 23: 240-244, 262.
Kenny, R.A. 1986. The Chinese restaurant syndrome: An anecdote revisited. Food Chem. Toxicol. 24: 351-354.
King, D.S. 1984. Psychological and behavioral effects of food and chemical exposure in sensitive individuals. Nutr. Health 3: 137-151.
Kjellman, N.I. and Bjorksten, B. 1997. Natural history and prevention of food hypersensitivity. In “Food Allergy: Adverse Reactions to Foods and Food Additives,” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon, pp. 445-459, Blackwell Science, Cambridge, Mass.
Kocian, J. 1988. Lactose intolerance. Int. J. Biochem. 20: 1-5.
Kolars, J.C., Levitt, M.D., Aouji, M., and Savaiano, D.A. 1984. Yogurt: An autodigesting source of lactose. N. Engl. J. Med. 310: 1-3.
Kulczycki, A. 1986. Aspartame-induced urticaria. Ann. Intern. Med. 104: 207-208.
Langeland, T. 1983. A clinical and immunological study of allergy to hen’s egg white. VI. Occurrence of proteins cross-reacting with allergens in hen’s egg white as studied in egg white from turkey, duck, goose, seagull, and in hen egg yolk, and hen and chicken sera and flesh. Allergy 38: 339-412.
Lehrer, S.B. 1986. The complex nature of food antigens: Studies of cross-reacting crustacea allergens. Ann. Allergy 57: 267-272.
Lehrer, S.B. and O’Neil, C.E. 1992. Occupational reactions in the food industry. Food Technol. 46(5): 153-156.
Lehrer, S.B., Helbling, A., and Daul, C.B. 1992. Seafood allergy: Prevalence and treatment. J. Food Safety 12: 61-76.
Lemke, P.J. and Taylor, S.L. 1994. Allergic reactions and food intolerances. In “Nutritional Toxicology,” ed. F.N. Kotsonis, M. Mackey, and J. Hjelle, pp. 117-137, Raven Press, N.Y.
Maeda, S., Orikaza, A., Kato, M., Mategi, Y., Shigeta, M., Tokuyama, K., Kurome, T., Naritomi, Y., Suehiro, K., and Kusabe, T. 1991. Eleven cases of anaphylaxis caused by grand keyhole limpet (abalone-like shellfish). Arerugi 40: 1415-1419.
Mager, J., Chevion, M., and Glaser, G. 1980. Favism. In “Toxic Constituents of Plant Foodstuffs,” 2nd Ed., ed. I.E. Liener, pp. 265-294, Academic Press, N.Y.
Marquardt, R.R. 1989. Vicine, convicine and their aglycones–divicine and isouramil. In “Toxicants of Plant Origin, Vol. II, Glycosides, ed. P.R. Cheeke, pp. 161-200, CRC Press, Boca Raton, Fla.
Martin, J.A., Compaired, J.A., de la Hoz, B., Quirce, S., Alonso, M.D., Igea, J.M., and Losada, E. 1992. Bronchial asthma induced by chick pea and lentil. Allergy 47: 185-187.
Mekori, Y.A. 1996. Introduction to allergic diseases. Crit. Rev. Food Sci. Nutr. 36S: S1-S18.
Metcalfe, D.D. 1984a. Food hypersensitivity. J. Allergy Clin. Immunol. 63: 749-762.
Metcalfe, D.D. 1984b. Diagnostic procedures for immunologically-mediated food sensitivity. Nutr. Rev. 42: 92-97.
Metcalfe, D.D., Astwood, J.D., Townsend, R., Sampson, H.A., Taylor, S.L., and Fuchs, R.L. 1996. Assessment of the allergenic potential of foods derived from genetically engineered crop plants. Crit. Rev. Food Sci. Nutr. 36S: S165-S186.
Muller, U., Weber, W., Hoffmann, A., Franke, S., Lange, R., and Vieths, S. 1998. Commercial soybean lecithins: A source of hidden allergens? Z. Lebensm. Unter. Forsch. 207: 341-351.
NFPA. 2001a. Food allergy issues alliance: Food allergen labeling guidelines. http://www.nfpa-food.org/science/FoodAllergenLabelingGuidelines.html. May, 22. National Food Processors Association, Washington, D.C.
NFPA. 2001b. Code of practice on managing food allergens. http://www.nfpa-food.org/news/040901%20CodeofPracticeBrochure.html. National Food Processors Association, Washington, D.C.
Nordlee, J.A., Taylor, S.L., Jones, R.T., and Yunginger, J.W. 1981. Allergenicity of various peanut products as determined by RAST inhibition. J. Allergy Clin. Immunol. 68: 376-382.
Nordlee, J.A., Taylor, S.L., Townsend, J.A., Thomas, L.A., and Bush, R.K. 1996. Identification of Brazil nut allergen in transgenic soybeans. N. Engl. J. Med. 334: 688-692.
Noyes, J.H., Boyd, G.K., and Settipane, G.A. 1979. Anaphylaxis to sunflower seed. J. Allergy Clin. Immunol. 63: 242-244.
O’Neil, C.E. and Lehrer, S.B. 1997. Occupational reactions to food allergens. In “Food Allergy: Adverse Reactions to Foods and Food Additives.” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon. Blackwell Science, Cambridge, Mass.
Ortolani, C., Ispano, M., Pastorello, E., Bigi, A., and Ansaloni, R. 1988. The oral allergy syndrome. Ann. Allergy 61: 47-52.
Paige, D.M., Bayless, T.M., Huang, S.S., and Wexler, R. 1975. Lactose hydrolyzed milk. Am. J. Clin. Nutr. 28: 818-822.
Pastorello, E. and Ortolani, C. 1997. Oral allergy syndrome. In “Food Allergy: Adverse Reactions to Foods and Food Additives,” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon, pp. 221-233, Blackwell Science, Cambridge, Mass.
Peters, S.P., Zangrilli, J.G., and Fish, J.E. 1998. Late phase allergic reactions. In “Allergy: Principles and Practice, Vol. I,” 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 342-355, Mosby, St. Louis, Mo.
Sakaguchi, M., Nakayama, T., and Inouye, S. 1996. Food allergy to gelatin in children with systemic immediatetype reactions, including anaphylaxis, to vaccines. J. Allergy Clin. Immunol. 98: 1058-1061.
Sakaguchi, M., Hori, H., Ebihara, T., Irie, S., Yanagida, M., and Inouye, S. 1999. Reactivity of the immunoglobulin E in bovine gelatin-sensitive children to gelatins from various animals. Immunol. 96: 286-290.
Sampson, H.A. 1988. The role of food allergy and mediator release in atopic dermatitis. J. Allergy Clin. Immunol. 81: 635-645.
Sampson, H.A. 1990. Food allergy. Curr. Opinion Immunol. 2: 542-547.
Sampson, H.A. 1991. Immunologic mechanisms in adverse reactions to foods. Immunol. Allergy Clin. No. Am. 11: 701-716.
Sampson, H.A. and McCaskill, C.M. 1985. Food hypersensitivity and atopic dermatitis: Evaluation of 113 patients. J. Pediatr. 107: 669-675.
Sampson, H.A., Mendelson, L., and Rosen, J. 1992. Fatal and near-fatal anaphylactic reactions to foods in children and adolescents. New Engl. J. Med. 327: 380-384.
Selner, J.C. and Staudenmayer, H. 1997. Food allergy: Psychological considerations. In “Food Allergy: Adverse Reactions to Foods and Food Additives,” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon, pp. 519-528, Blackwell Scientific, Oxford.
Sicherer, S.H., Munoz-Furlong, A., Burks, A.W., and Sampson, H.A. 1999. Prevalence of peanut and tree nut allergy in the U.S. determined by a random digit dial telephone survey. J. Allergy Clin. Immunol. 103: 559-562.
Simons, F.E.R. 1998. Antihistamines. In “Allergy: Principles and Practice, Vol. I,” 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 612-637, Mosby, St. Louis, Mo.
Simoons, F.J. 1980. Age of onset of lactose malabsorption. Pediatrics 66: 646-648.
Skerritt, J.H., Devery, J.M., and Hill, A.S. 1990. Gluten intolerance: Chemistry, celiac-toxicity, and detection of prolamins in foods. Cereal Foods World 35: 638-644.
Sloan, A.E. 1986. A perspective on popular perceptions of adverse reactions to foods. J. Allergy Clin. Immunol. 78: 127-132.
Solomon, W.R. and Platts-Mills, T.A.E. 1998. Aerobiology and inhalant allergens. In “Allergy: Principles and Practice, Vol. I,” 5th Ed., ed. E. Middleton, Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson, Jr., J.W. Yunginger, and W.W. Busse, pp. 367-403, Mosby, St. Louis, Mo.
Stevenson, D.D. and Simon, R.A. 1981. Sensitivity to ingested metabisulfites in asthmatic subjects. J. Allergy Clin. Immunol. 68: 26-32.
Stevenson, D.D., Simon, R.A., Lumry, W.R., and Mathison, D.A. 1986. Adverse reactions to tartrazine. J. Allergy Clin. Immunol. 78: 182-191.
Stratton, J.E. and Taylor, S.L. 1991. Scombroid poisoning. In “Microbiology of Marine Food Products,” ed. D.R. Ward and C. Hackney, pp. 331-351, Van Nostrand Reinhold, N.Y.
Stratton, J.E., Hutkins, R.W., and Taylor, S.L. 1991. Biogenic amines in cheese and other fermented foods: A review. J. Food Prot. 54: 460-469.
Strobel, S. 1997. Oral tolerance: Immune responses to food antigens. In “Food Allergy: Adverse Reactions to Foods and Food Additives,” 2nd Ed., ed. D.D. Metcalfe, H.A. Sampson, and R.A. Simon, pp. 107-135, Blackwell Science, Boston, Mass.
Strober, W. 1986. Gluten-sensitive enteropathy: A nonallergic immune hypersensitivity of the gastrointestinal tract. J. Allergy Clin. Immunol. 78: 202-211.
Suarez, F.L. and Savaiano, D.A. 1997. Diet, genetics, and lactose intolerance. Food Technol. 51: 74-76.
Sumner, S.S., Speckhard, M.W., Somers, E.B., and Taylor, S.L. 1985. Isolation of histamine-producing Lactobacillus buchneri from Swiss cheese implicated in a food poisoning outbreak. Appl. Environ. Microbiol. 50: 1094-1096.
Tarasoff, L. and Kelly, M.F. 1993. Monosodium L-glutamate: A double-blind study and review. Food Chem. Toxicol. 31: 1019-1035.
Taylor, S.L. 1986. Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 17: 91-128.
Taylor, S.L. 1987. Allergic and sensitivity reactions to food components. In “Nutritional Toxicology,” Vol. 2, ed. J.N. Hathcock, pp. 173-198, Academic Press, N.Y.
Taylor, S.L. 1990. Food allergies and related adverse reactions to foods: A food science perspective. In “Food Allergies and Adverse Reactions,” ed. J.E. Perkin, pp. 189-206, Aspen Publishers, Rockville, Md.
Taylor, S.L. 1996. Genetically-engineered foods: Commercial potential, safety, and allergenicity. Food Australia 48: 308-311.
Taylor, S.L. and Dormedy, E.S. 1998. The role of flavoring substances in food allergy and intolerance. Adv. Food Nutr. Res. 42: 1-44.
Taylor, S.L. and Hefle, S.L. 2000a. Good manufacturing practices for allergenic foods: Use of shared equipment. Food Allergy and Intolerance 1(1): 47-50.
Taylor, S.L. and Hefle, S.L. 2000b. Good manufacturing practices for the food industry: Use of rework. Food Allergy and Intolerance 1(2): 114-117.
Taylor, S.L. and Hefle, S.L. 2000c. Good manufacturing practices for the food industry: Suppliers and co-packers. Food Allergy and Intolerance 1(3): 208-213.
Taylor, S.L. and Hefle, S.L. 2001a. Assessing the allergenicity of foods produced through agricultural biotechnology: A new decision tree approach. Food Allergy Intol. 2: 149-156.
Taylor, S.L. and Hefle, S.L. 2001b. Good manufacturing practices for allergenic foods: Packaging and labeling strategies. Food Allergy and Intolerance 2(1): 53-58.
Taylor, S.L. and Lehrer, S.B. 1996. Principles and characteristics of food allergens. Crit. Rev. Food Sci. Nutr. 36S: S91-S118.
Taylor, S.L., Guthertz, L.S., Leatherwood, M., Tillman, F., and Lieber, E.R. 1978. Histamine production by foodborne bacterial species. J. Food Safety 1: 173-187.
Taylor, S.L., Busse, W.W., Sachs, M.I., Parker, J.L., and Yunginger, J.W. 1981. Peanut oil is not allergenic to peanut-sensitive individuals. J. Allergy Clin. Immunol. 68: 372-375.
Taylor, S.L., Keefe, T.J., Windham, E.S., and Howell, J.F. 1982. Outbreak of histamine poisoning associated with consumption of Swiss cheese. J. Food Prot. 45: 455-457.
Taylor, S.L., Bush, R.K., and Busse, W.W. 1986a. Avoidance diets: How selective should we be? N. Engl. Reg. Allergy Proc. 7: 527-532.
Taylor, S.L., Higley, N.A., and Bush, R.K. 1986b. Sulfites in foods: Uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity and hypersensitivity. Adv. Food Res. 30: 1-76.
Taylor, S.L., Bush, R.K., Selner, J.C., Nordlee, J.A., Wiener, M.B., Holden, K., Koepke, J.W., and Busse, W.W.1988. Sensitivity to sulfited foods among sulfite-sensitive subjects with asthma. J. Allergy Clin. Immunol. 81: 1159-1167.
Taylor, S.L., Stratton, J.E., and Nordlee, J.A. 1989a. Histamine poisoning (scombroid fish poisoning): An allergy-like intoxication. J. Toxicol. Clin. Toxicol. 27: 225-240.
Taylor, S.L., Nordlee, J.A., and Rupnow, J.H. 1989b. Food allergies and sensitivities. In “Food Toxicology: A Perspective on the Relative Risks, ed. S.L. Taylor and R.A. Scanlan, pp. 255-295, Marcel Dekker, N.Y.
Taylor, S.L., Hefle, S.L., and Munoz-Furlong, A. 1999. Nutrition and the life cycle: Food allergies and avoidance diets. Nutr. Today 34 (1): 15-22.
Taylor, S. L. and Hefle, S. L. 2001. Will genetically modified foods be allergenic? J. Allergy Clin. Immunol. 107:765-771.
Van Asperen, P.P., Kemp, A.S., and Mellis, C.M. 1983. Immediate food hypersensitivity reactions on the first known exposure to food. Arch. Dis. Child. 58: 253-256.
van Ree, R. and Aalberse, R.C. 1993. Pollen-vegetable food crossreactivity: Serological and clinical relevance of crossreactive IgE. J. Clin. Immunoassay 16: 124-130.
Vandenplas, Y., Hauser, B., Van den Borre, C., Sacre, L., and Dab, I. 1992. Effect of a whey hydrolysate prophylaxis of atopic disease. Ann. Allergy 68: 419-424.
Wuthrich, B., Stager, J., and Johansson, S. 1990. Celery allergy associated with birch and mugwort pollinosis. Allergy 45: 566-571.
Yunginger, J.W., Sweeney, K.G., Sturner, W.Q., Giannandrea, L.A., Tiegland, J.D., Bray, M., Benson, P.A., York, J.A., Biedrzycki, L., Squillace, D.L., and Helm, R.M. 1988. Fatal food-induced anaphylaxis. J. Am. Med. Assoc. 260: 1450-1452.
Zeiger, R.S. and Heller, S. 1995. The development and prediction of atopy in high-risk children: Follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J. Allergy Clin. Immunol. 95: 1179-1190.
