Activated Lactoferrin—A New Approach to Meat Safety
Activated form of natural antimicrobial improves safety of beef and poultry by preventing attachment and growth of bacteria.
Activated lactoferrin (ALF) is a new form of a naturally occurring protein from milk that acts as a powerful deterrent to pathogenic bacteria that may be present on a meat surface. Considered generally recognized as safe (GRAS) by the Food and Drug Administration and recently approved by the U.S. Dept. of Agriculture for use on fresh beef, ALF can be sprayed onto carcasses to help prevent bacterial contamination during processing or can be applied to a subprimal or finished beef surface prior to final packaging to inhibit bacterial growth and extend shelf life. This article discusses the science behind the patented technology and its potential applications.
Lactoferrin
The occurrence of natural antimicrobial agents in milk, eggs, plants, probiotics, salts, and acids has been well recognized for centuries, but the structure–function relationship of such bioactive compounds has been scientifically proven only in recent years.
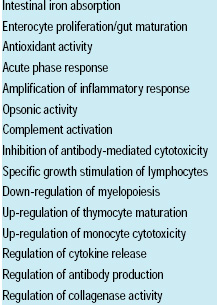 One such antimicrobial agent is lactoferrin (Fig. 1), a major bioactive glycoprotein in milk, saliva, tears, seminal fluids, mucins, and the secondary granules of neutrophils. Its ability to bind two Fe+3 ions with high affinity, in cooperation with two HCO3 – ions, contributes to its major structure–functional properties, including antimicrobial activity. It plays an important regulatory role in various physiological pathways (Table 1) and is considered a major component of the preimmune innate defense in mammals (Naidu, 2000). These multi-functional attributes make LF a potent nutraceutical and an effective natural food antimicrobial system.
One such antimicrobial agent is lactoferrin (Fig. 1), a major bioactive glycoprotein in milk, saliva, tears, seminal fluids, mucins, and the secondary granules of neutrophils. Its ability to bind two Fe+3 ions with high affinity, in cooperation with two HCO3 – ions, contributes to its major structure–functional properties, including antimicrobial activity. It plays an important regulatory role in various physiological pathways (Table 1) and is considered a major component of the preimmune innate defense in mammals (Naidu, 2000). These multi-functional attributes make LF a potent nutraceutical and an effective natural food antimicrobial system.
The antimicrobial functionality of LF is dependent on its protein conformation and milieu conditions (Naidu and Arnold, 1997). Bacteriostasic effect is enhanced when LF binds to a microbial cell surface (Dalamastri et al., 1988; Naidu et al., 1993). Accordingly, specific LF-binding microbial targets exist in a variety of Gram-positive and Gram-negative bacteria (Naidu et al., 1991, 1992, 1993). The high-affinity interaction of LF with pore-forming outer-membrane proteins (OMPs) of Gram-negative enterics, including Escherichia coli, is critical for the antimicrobial outcome of LF (Gado et al., 1991; Tigyi et al., 1992; Erdei et al., 1993; Naidu and Arnold, 1997). LF-mediated outer-membrane damage in Gram-negative bacteria (Ellison et al., 1988) and the LF-induced antibiotic potentiation by causing altered permeation (Naidu and Arnold, 1994) are examples of such antimicrobial outcomes.
--- PAGE BREAK ---
The interaction of LF with microbial surfaces—OMPs of Gram-negative bacteria in particular—has led to other antimicrobial mechanisms, such as the inhibition of microbial attachment to sub-epithelial matrix proteins and detachment of bacteria from mucosal surfaces. Intracellular events, such as blocking of microbial attachment factors such as fimbriae (hairlike structures) and other adhesins (putative receptors), have been observed (Naidu and Bidlack, 1998). Furthermore, the immobilization of LF to mucosal surfaces containing sulfated glycans such as heparan sulfate or its closely related analogs, including galactose-rich polysaccharide (a water-soluble fraction from agar) and carrageenans, were found to enhance the antimicrobial spectrum of LF multi-fold.
Though credited with an impressive list of nutraceutical benefits, certain factors limit the functionality of LF after isolation from milk. Protein separation conditions always pose the risk of denaturation or structural alteration of the LF molecule. The isolation process may even generate cationic peptides as degradation products. These cationic peptides can also be generated by enzymatic activity (Tomita et al., 1994). The highly cationic N-terminus region of LF could facilitate charge-induced protein aggregation and inactivate the molecule. Also, the isolated LF is highly susceptible to conformational changes, thermal uncoiling, and proteolysis. Furthermore, milieu conditions such as pH, ionic environment with elevated calcium or phosphates, iron excess, and improper citrate/bicarbonate ratios could markedly diminish the antimicrobial activity of LF. Therefore, development of a technology to overcome these limitations is critical.
Activated Lactoferrin
Exocrine secretions, including milk, deliver LF in a free form, and this molecule interacts with an array of substrates on epithelial milieu such as glycosaminoglycans (heparan sulfate in mucins), specific receptors on intestinal brush borders, and DNA on crypt cells (Davidson and Lönnerdal, 1988; Nichols et al., 1990; Wu et al., 1995). The multifunctional pathways of LF are triggered following a specific immobilization (binding) of this protein molecule to various cellular targets, including neutrophils, phagocytes, and platelets. This immobilization-mediated activation of LF with eukaryotic cell cascade is achieved via the N-terminus region of the protein.
Immobilization of LF via its N-terminus region, neutralization of cationic peptides by salt, optimization of milieu by adjusting pH and citrate/bicarbonate ratio, and establishing an equilibrium between bound (immobilized) vs unbound LF formed the basis for in-vitro activation of LF. Accordingly, a patented technology (Naidu, 2001) was developed to produce ALF.
In this process, milk LF is immobilized on a food-grade glycosaminoglycan such as galactose-rich polysaccharide or carrageenan, solubilized in a precalibrated citrate/bicarbonate buffer system containing sodium chloride and an excess of unbound LF. The result is a true microbial blocking agent that interferes with adhesion/colonization, detaches live or dead microorganisms from biological surfaces, inhibits microbial growth/multiplication, and neutralizes the activity of endotoxins.
--- PAGE BREAK ---
• Microbial Adhesion Blocking. Microbial attachment to epithelial mucosa or biosurfaces is the initial step in the pathogenesis of many infections as well as spoilage of foods. Biointeractions involving ligands/adhesins with specific receptors and nonspecific binding mechanisms (electrostatic, hydrophobic, and van der Waals forces) contribute to the adhesion/colonization of microorganisms to biosurfaces. Enteric bacteria, E. coli in particular, harbor various fimbria on their cell surface to promote bacterial adhesion and colonization on the mucosal surface (Parry and Rooke, 1985). For example, enterohemorrhagic E. coli O157:H7 adheres tightly to epithelial surfaces (Louie et al., 1993) and to collagens on beef tissue (Fig. 2). Several other foodborne enteropathogens possess specific cell-surface appendages such as fimbria or colonization factor antigens that facilitate their anchoring to host-tissue-matrix components such as fibronectin, collagens, and mucins (Höök et al., 1989).
Specific binding of ALF to OMPs of Gram-negative bacteria leads to inhibition of various cellular functions and deregulation of adhesin/fimbrial synthesis on the bacterial surface (Fig. 3). Furthermore, ALF–OMP interaction can also cure plasmids by diffusion, resulting in the loss of colonization-factor antigens and certain enterotoxins in E. coli.
• Bacterial Detachment. Binding to tissue-matrix components such as collagens, mucins, and fibronectin is a pivotal anchor mechanism for many bacteria. ALF also binds to the same anchor sites on tissue surfaces with a greater affinity. Therefore, ALF could block tissue interaction of bacteria by competitive binding-inhibition. In binding-displacement studies, ALF at low molar concentration was shown to detach tissue-bound bacteria, either viable or dead (debris).
• Microbial Growth Inhibition. The ability of iron to alternate between two valence states, Fe+2 and Fe+3, is the most important biological property, evident in many pathways of bioenergy synthesis, including the electron transport system that forms ATP by phosphorylation. Thus, iron deprivation leads to conservation of bioenergy and inhibition of cellular multiplication. Accordingly, various iron-binding proteins have evolved in the animal physiological system to sequester iron from the milieu.
Thus, the reversible transition of LF between iron-deficient (apo-form) to iron-sufficient (holo-form) states is the key mechanism for antimicrobial activity. Also, the pathogenesis of bovine mastitis, the udder disease in LF-rich milk environment, emphasized the critical role of citrate/bicarbonate ratios (Nonnecke and Smith, 1984). Furthermore, LF binding to the microbial surface results in increased antimicrobial activity (Dalamastri et al., 1988; Naidu et al., 1993). This interaction could be enhanced by reduction or neutralization of the net surface charge (milieu near pI) on the LF molecule.
All the above function-limiting factors, both molecular and milieu, augmentory as well as inhibitory, are controlled in the compositional design of the ALF. Accordingly, the citrate/bicarbonate ratios are calibrated in the ALF formulation to facilitate optimal apo/holo transition. Salts and pH are adjusted in ALF to reduce cationic charge interactions. And an immobilization step is included to stabilize LF by reorganizing its molecular epitopes, thereby augmenting the MBA function, a structure-related activity. ALF is also markedly resistant to proteolytic degradation and thermal uncoiling of its polypeptide chain.
Various growth-inhibition activities, individual and synergistic, reported for LF, are also exhibited by ALF, but with a multi-fold potentiation over the original effect. Accordingly, the minimal inhibitory concentrations required for ALF are significantly lower than those for LF. In contrast to LF, ALF demonstrates a potent antimicrobial activity in iron-rich milieu such as meats and biological fluids. ALF also shows synergism with bacteriocins (from probiotics), colicins (from E. coli), and salivary histatins.
--- PAGE BREAK ---
• Antiviral Activity. ALF also exhibits broad-spectrum activity against both DNA and RNA viruses. Its ability to interact with nucleic acids and its capacity to bind eukaryotic cells and prevent viral adhesion seem to be the possible antiviral mechanisms. Ongoing studies also indicate the effectiveness of ALF in the control of biofilms on nonbiological surfaces such as food processing equipment.
Efficacy Testing
The antimicrobial activity of ALF against E. coli O157:H7 has been tested and compared to that of LF. Studies were performed independently at a state university in California, a national food research institute in the United Kingdom, and the Center of Expertise for Nutrition, DMV International, Wageningen, the Netherlands.
The efficacy of ALF and LF to detach collagen-bound E. coli O157:H7 was measured by an in-vitro adhesion-blocking assay, which showed a potent adhesion-blocking effect against collagen-bound E. coli O157:H7. The bacterial detachment efficacy was 2.7 log higher than the LF treatment.
Efficacy of ALF and LF on the growth inhibition of E. coli O157:H7 was tested by measuring impedence change in typticase soy broth (TSB) using a microbial monitoring system (Bactometer, bioMerieux, Hazelwood, Mo.). Stasis was also monitored as the turbidity change in TSB (optical density reading at 600 nm) using a micro-plate reader (VersaMax, Molecular Devices, Sunnyvale, Calif.). ALF caused a 17.4-hr greater stasis effect than LF against E. coli. The optical density data indicated that the minimal inhibitory concentrations (24-hr stasis) for 1% LF and 1% ALF against a 4-log inoculum of E. coli O157:H7 were >1,000 μg/mL and 62 μg/mL, respectively.
Efficacy studies on growth inhibition were also performed on beefsteaks sprayed with 1% LF or 1% ALF. A pre-marked 1-in2 meat surface was inoculated with about 4-log cells of E. coli O157:H7, and the samples were left at room temperature for 24 hr. The ALF spray demonstrated a 2.5-log greater efficacy in causing stasis of E. coli O157:H7 compared to the LF spray.
Combinations of interventions such as acid rinses, hot/cold water washes, and steam pasteurization are currently used as safety hurdles in beef processing. A digitally simulated spray (DSS) system was designed to test the efficacy of current interventions with or without ALF spray to decontaminate E. coli O157:H7 from beef.
The DSS system consists of a programmable conveyor line that carries meat through 12 processing chambers—6 spray chambers, 5 pause chambers, and 1 meat-loading chamber. The spray chambers are connected to individual 2-gal delivery tanks via digitally controlled pumps with adjustable spray and pause time. The 6 spray chambers are hosed into individual fluid collectors. A sterile stainless-steel meat-loading frame is placed in a beta-ray-shielded acrylic box, and a beef sample (about 4 in2) is hooked in the center of the frame. A sterile bactainer (an open stainless-steel hollow with a sharp-edged end 1-in square) is firmly pressed into the meat.
The meat surface exposed inside the bactainer was inoculated with 5 mL of 3H-thymidine-labeled E. coli O157:H7 (~ 5 x 107 cells) and kept for 2 hr at room temperature. The bactainer was then removed, and the loading frame with E. coli-infected meat was removed from the acrylic box and mounted on the meat-loading chamber of the DSS. Samples were subjected to a sanitizing treatment typical of commercial beef processing. The treatment consisted of five spray-wash steps: cold water (10 sec), 2% lactic acid (10 sec), hot water (180ºF for 30 sec), cold water (10 sec), and 2% lactic acid (10 sec). Samples were also treated using an additional 10-sec wash step with 1% ALF.
After treatment, the loading frame was dismounted and placed in the beta-ray-shielded acrylic box. Six random samples (about 0.5 g) were excised from the tissue inoculated with E. coli. Samples were digested with 4 mL of tissue homogenizer overnight at 55ºC in a waterbath-shaker. Then 10 mL of scintillation cocktail was added to the homogenate, and the radioactivity (disintegrations/min, DPM) was measured in a scintillation counter.
The regular sanitizing assembly averaged 72.2% of E. coli detachment/g of beef tissue. The sanitizing assembly combined with the 1% ALF spray demonstrated almost 100% efficacy—an average 99.9% E. coli detachment/g of beef tissue—in three experimental runs. Acid rinses and hot water washes seemed to reduce E. coli O157:H7 to a significant level, but scanning electron microscopy of the processed beef revealed that most of the bacterial debris remained firmly attached to the tissue (Fig. 4A). An additional 10-sec spray/rinse with 1% ALF effectively sanitized the contaminated beef surface by removing debris and residual bacteria (Fig. 4B).
--- PAGE BREAK ---
Sensory Testing
Sensory studies were conducted at Oklahoma State University, Stillwater. Strip loin samples with ALF treatment and their paired controls without any treatment were vacuum packaged and allowed to age at refrigeration temperature below 38ºF for 14, 21, 28, or 35 days. Then each strip loin was fabricated into 1-in-thick steaks, and random samples were again treated with ALF. Each sample was sealed in optimal modified-atmosphere packaging (80% O2 and 20% CO2) and kept in commercial display cases for 14 days under cool-white fluorescent light at 32–36ºF.
Samples were tested twice daily by a trained panel for lean color, fat color, percentage discoloration, and overall appearance. Trained panelists conducted palatability and sensory analysis, including tenderness (Warner-Bratzler shear force test), juiciness, cooked beef flavor, and overall acceptability of steak samples. Lipid oxidation on the meat surface was estimated by the TBA test. Finally, total plate counts were performed to determine microbiological keeping quality.
The results indicated that ALF treatment could extend the retail display life by 1.7–2.5 days for case-ready packaged steaks compared to non-treated steaks in conventional packages. ALF-treated and control samples showed no differences in tenderness, juiciness, or off-flavor of cooked beef, and the panelists preferred the flavor of the ALF-treated samples. Subprimals stored for 21 days following ALF application exhibited a 5-fold reduction in total microbial plate counts compared to non-treated steaks.
Regulatory and Commercial Status
LF occurs naturally in milk, milk-derived ingredients and products, and, to a lesser extent, in beef tissue. Thus, persons who consume milk or milk-derived ingredients or beef already consume LF. Milk-derived ALF is considered GRAS by the Food and Drug Administration [21 CFR.170.36(f)]. It is permitted at levels of 65.2 mg/kg of beef, according to FDA’s directive of October 23, 2001. The U.S. Dept. of Agriculture approved use of ALF on fresh beef in December 2001. For other countries, the status of ALF will need to be confirmed, but the following is the regulatory status of LF.
Currently, the European Union does not have a specific regulation for LF. The EU has a directive 83/417/EEC for the use of protein derived from milk, but LF could be included in this directive when it attains regulated status. LF is considered a milk protein (provision 79/112/EEC allows all types of milk protein to be labeled as milk protein) and hence would be allowed in foods.
In Japan, LF is specified in the List of Existing Food Additives, which is a list of the permitted natural additives. In South Korea, LF concentrates are also listed as authorized natural additives. In Taiwan, LF may be used in special nutritional foods under the condition “only for supplementing foods with an insufficient nutritional content and may be used in appropriate amounts according to actual requirements.”
ALF will be commercially produced and marketed by aLF Ventures, LLC, a U.S.-based partnership between Farmland National Beef Packing Co., L.P., Kansas City, Mo., and DMV International Nutritionals, one of the largest producers of LF worldwide. Farmland, the fourth-largest beef packing company in the U.S., expects to be the first company to utilize ALF commercially, once final application systems development and testing are completed.
ALF is manufactured from bovine LF extracted from cheese whey or skim milk. Other ingredients in the formulation—sodium bicarbonate, citric acid, sodium chloride, and carrageenan—are commercially available food-grade chemicals. The aqueous ALF formulation is constituted in deionized water at concentrations ranging from 1 to 4%, depending on the application requirement.
ALF has been shown to be effective when applied to beef carcasses as an additional intervention step or to the subprimal at fabrication, using the system shown in Fig. 5. ALF is delivered onto the meat surface as a fine mist, using electrostatic or high-pressure liquid spray nozzles. The flow pattern and spray time are digitally monitored by a programmable logic controller (PLC) for uniform coverage of ALF on the meat surface at optimum functional concentration. A solid-phase immunoassay based on a fluorescent-labeled LF-antibody has been developed to verify the coverage and quantify ALF-coating on the meat surface.
--- PAGE BREAK ---
Future Possibilities
ALF has demonstrated microbial blocking activity against a variety of foodborne pathogens, including E. coli O157:H7, Listeria monocytogenes, Salmonella spp., Campylobacter spp., Vibrio spp., Aeromonas hydrophila, and Staphylococcus aureus, as well as the food spoilage microorganisms Bacillus spp., Pseudomonas spp., and Klebsiella spp. ALF also inhibits yeast and molds, as well as DNA and RNA viruses. These functional attributes make ALF a potential microbial intervention for poultry, pork, fish/seafood, and produce processing applications.
ALF is also effective in the detachment of multi-drug-resistant Salmonella Typhimurium DT104, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus from biosurfaces. Furthermore, ALF could inhibit radiation-resistant bacteria such as Brochothrix thermospacta, Deinococcus radiopugnans, Deinococcus radiodurans, Acinetobacter radioresistens, and Methylobacterium radiotolerans. Studies are underway to evaluate the antimicrobial efficacy of ALF incorporated into edible food packaging materials and sausage casings. Finally, the synergism with anti-biofilm treatments makes ALF a powerful sanitizing agent in food processing.
A Potent Antimicrobial System
Most antimicrobials in use today kill microorganisms and leave debris as well as potent toxins on processed foods. In contrast, ALF is an extremely powerful antimicrobial intervention that prevents microbial proliferation and attachment on biosurfaces such as beef or poultry tissue. The ability to detach the leftover microbial debris and to inactivate the surface-splattered endotoxins makes ALF a highly effective intervention all by itself or as an additional step in current multi-hurdle sanitizing systems.
In the ever-competitive food market, where consumer demand is steadily increasing for minimally processed foods that sustain functionality of naturally occurring bioactive ingredients, ALF clearly stands out as a potent natural antimicrobial system with an impressive list of well-documented multi-functional properties and nutraceutical benefits.
by A. Satyanarayan Naidu
The author, a Professional Member of IFT, is Director, N-terminus Research Laboratory, 981 Corporate Center Dr., #110, Pomona, CA 91768.
Edited by Neil H. Mermelstein,
Editor
References
Dalmastri, C., Valenti, P., Visca, P., Vittorioso, P., and Orsi, N. 1988. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica 11: 225-230.
Davidson, L., and Lönnerdal, B. 1988. Specific binding of lactoferrin to brush border membrane: Ontogeny and effect of glycan chain. Am. J. Physiol. 257: 930-934.
Ellison, R.T., Giehl, T.J., and LaForce, F.M. 1988. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 56: 2774-2781.
Erdei, J., Forsgren, A., and Naidu, A.S. 1994. Lactoferrin binds to porins OmpF and OmpC in Escherichia coli. Infect. Immun. 62: 1236-1240.
Gado, I., Erdei, J., Laszlo, V.G., Paszti, J., Czirok, E., Kontrohr, T., Toth, I., Forsgren, A., and Naidu, A.S. 1991. Correlation between human lactoferrin binding and colicin susceptibility in Escherichia coli. Antimicrob. Agents Chemother. 35: 2538-2543.
Höök, M., Switalski, L., Wadström, T., and Lindberg, M. 1989. Interactions of pathogenic microorganisms with fibronectin. In “Fibronectin,” ed. D.F. Mosher, pp. 295-308. Academic Press, New York.
Louie, M., de Azavedo, J.C,, Handelsman, M.Y., Clark, C.G., Ally, B., Dytoc, M., Sherman, P., and Brunton, J. 1993. Expression and characterization of the eaeA gene product of Escherichia coli serotype O157: H7. Infect. Immun. 61: 4085-4092.
Naidu, A.S. 2000. Lactoferrin. In “Natural Food Antimicrobial Systems,” ed. A.S. Naidu, pp.17-102. CRC Press, Boca Raton.
Naidu, A.S. 2001. Immobilized lactoferrin antimicrobial agents and the use. U.S. patent 6,172,040 B1.
Naidu, A.S. and Arnold, R.R. 1994. Lactoferrin interaction with salmonellae potentiates antibiotic susceptibility in vitro. Diagn. Microbiol. Infect. Dis. 20: 69-75.
Naidu, A.S. and Arnold, R.R. 1997. Influence of lactoferrin on host-microbe interactions. In “Lactoferrin—Interactions and Biological Functions,” ed. T.W. Hutchens and B. Lönnerdal, pp. 259-275. Humana Press, Totowa, N.J.
Naidu, A.S. and Bidlack, W.R. 1998. Milk lactoferrin—Natural microbial blocking agent (MBA) for food safety. Environ. Nutr. Interact. 2: 35-50.
Naidu, S.S., Erdei, J., Czirok, E., Kalfas, S., Gado, I., Thoren, A., Forsgren, A., and Naidu, A.S. 1991. Specific binding of lactoferrin to Escherichia coli isolated from human intestinal infections. APMIS 99: 1142-1150.
Naidu, A.S., Andersson, M., and Forsgren, A. 1992. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J. Med. Microbiol. 36: 177-183.
Naidu, S.S., Svensson, U., Kishore, A.R., and Naidu, A.S. 1993. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob. Agents Chemother. 37: 240-245.
Nichols, B.L., McKee, K. L., and Huebers, H. A. 1990. Iron is not required in the lactoferrin stimulation of thymidine incorporation into the DNA of rat crypt enterocytes. Pediatr. Res. 27: 525-528.
Nonnecke, B.J. and Smith, K.L. 1984. Biochemical and antibacterial properties of bovine mammary secretion during mammary involution and at parturition. J. Dairy Sci. 67: 2863-2872.
Parry, S.H. and Rooke, D.M. 1985. Adhesins and colonization factors of Escherichia coli. In “The Virulence of Escherichia coli—Reviews and Methods,” ed. M. Sussman, pp. 79-155. Academic Press, London.
Tigyi, Z., Kishore, A.R., Maeland, J.A., Forsgren, A., and Naidu, A.S. 1992. Lactoferrin-binding proteins in Shigella flexneri. Infect. Immun. 60: 2619-2626.
Tomita, M., Takase, M., Wakabayashi, H., and Bellamy, W. 1994. Antimicrobial peptides of lactoferrin. Adv. Exp. Med. Biol. 357: 209-218.
Wu, H.F., Monroe, D.M., and Church, F.C. 1995. Characterization of the glycosaminoglycan binding region of lactoferrin. Arch. Biochem. Biophys. 317: 85-92.
