The Vacuum/Steam/Vacuum Process
Prototype process reduces bacteria on solid foods without thermal damage.
Numerous research efforts are underway to eliminate surface bacteria from food. Some attempt to sanitize the surface with aqueous solutions of chemicals such as lactic acid. Some incorporate the antimicrobial agents into the product or packaging. In this manner, they kill the bacteria and leave a residual antimicrobial agent in the product to prevent further recontamination. Some of these agents are commercially available.
There are also thermal processes. One is a commercially available post-packaging hot-water process in which hot dogs are submerged in hot water near boiling for 20 min. Other processed meats are submerged for longer times. The process is effective but is large, requires a higher-cost packaging film, and requires a package-drying step. The process is generally suitable for cooked product.
Various steam processes are under development for killing bacteria on the surface of solid foods. Bremer et al. (2002) developed a pilot-plant process using steam to kill pathogens on the skin, gut, and gill cavities of king salmon. The process uses 689 kPa steam for an 8-sec hold time. Under these conditions, they achieved a 4-log reduction of Listeria monocytogenes.
The Food Refrigeration and Process Engineering Research Centre at the University of Bristol has been working on several processes, including a steam process to surface-treat foods (Anonymous, 1999). They have looked at steam from subatmospheric to pressurized. Times ranged from 10 to 40 sec at 85ºC. Reductions were 1–4 log on beef. They are also investigating the use of hot air, water immersion, infrared, microwaves, and ozone.
Frigoscandia developed and patented a process based on atmospheric steam to kill bacteria on the surface of beef carcasses (Wilson et al., 1999, 2000). The carcasses pass between spray nozzles that shower the steam onto the surface for 8 sec. This process reduces the bacteria level by more than 1 log (Phebus et al., 1997; Nutsch et al., 1997).
The VSV Concept
If we look at solid foods up close, the surface will appear quite rough with many pores (Fig.1). It’s difficult to kill bacteria that get into these pores with sanitizing solutions because surface tension prevents the liquid from entering the pores.
Steam can rapidly kill bacteria. It is a gas, and its mean free path is less than the size of bacteria (Morgan et al., 1996a). Therefore, steam should be able to enter the pores and kill the bacteria.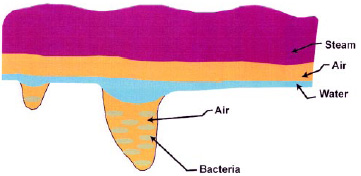
However, we live in an ocean of air that contains a varying amount of water vapor (humidity), so that a very thin layer of air plus the entrained moisture surrounds all solid food. Some solid food, such as chicken, is processed in water and is wet. Steam cannot pass through these barriers of air and water to reach the bacteria. These barriers act as insulation against the steam. Steam must transfer its energy across these barriers to kill the bacteria. The time to transfer the energy across the barriers is too long and causes thermal damage to sensitive foods like chicken. So how can we get the steam rapidly into intimate contact with the bacteria without thermally damaging the product?
--- PAGE BREAK ---
It is critical that the thermal transfer to the bacteria be extremely fast so that only the bacteria are affected, not the food. Morgan et al. (1996a, b) proposed the following concept to remove these barriers to thermal transfer: Apply vacuum to the food to remove the air and moisture; rapidly apply steam to kill the bacteria in the pores; then expose the food to vacuum again to remove the condensate and evaporatively cool the surface. The times of exposure must be short, on the order of 0.1 sec.
Normally, steam condenses as a film (film-type condensation), but under special circumstances it can condense as droplets (dropwise condensation). In the latter case, the surface is not uniformly wetted and the condensate appears as discrete droplets. Heat transfer coefficients for dropwise condensation can be 6–18 times higher than for film-type condensation. The treatment proposed by Morgan et al., in addition to removing the insulating films of water and air, could also be promoting dropwise condensation.
Both explanations of the mechanisms are hypotheses. We are currently investigating methods to elucidate the mechanism. In theory, the concept is simple. In practice, it’s not so simple to achieve.
Pilot-Plant Process Developed
We developed a pilot-plant process, based on this concept, that exposes solid food products to vacuum, then steam, then vacuum again. Saturated steam is used to take advantage of the very large latent heat of condensation relative to the sensible heat transferred due to temperature difference in cooling superheated steam. We call the process the Vacuum/Steam/ Vacuum (VSV) process. Fig. 2 shows the pilot-plant VSV processor.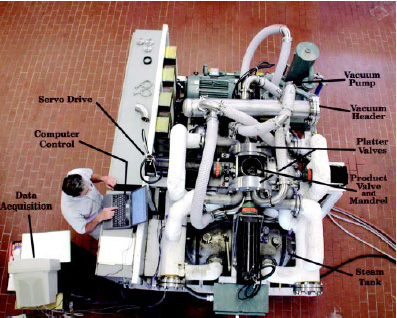
The initial and primary focus of the research was on chicken. Several large chicken processors cooperated in testing the process on freshly slaughtered chicken carcasses, but there was no statistically significant bacterial kill. The prototype process did not work for whole carcasses. Why?
After a series of studies, it became apparent that there was no effective exposure to vacuum and steam in the cavity. Retrofitting various mandrels to the main chamber to expose the cavity to vacuum and steam showed that the mandrel concept could work if perfected. The VSV process was applied to whole carcasses using three different mandrels. Saturated steam at 138°C, steam time of 0.1 sec, and vacuum time of 0.5 sec were used. Mandrel 1 yielded a 0.72-log cfu/mL kill, mandrel 2 a 0.70-log cfu/mL kill, and mandrel 3 a 0.79-log cfu/mL kill, all statistically significant at the 95% confidence level (Kozempel et al., 2001a). This showed conclusively that adding a mandrel to the VSV processor gave statistically significant bacteria kills and that the cavity was treated.
--- PAGE BREAK ---
Field Testing
A new VSV processor (Fig. 3) was designed to be mobile to go to the processors to evaluate its capability with freshly slaughtered carcasses. The unit mounts on a flatbed truck and is self-sufficient with all utilities needed. A detailed description of the mobile VSV processor can be found in Kozempel et al. (2003).
The mobile unit was first tested in the pilot plant. To determine the effectiveness of the process to kill bacteria, carcasses were inoculated with nonpathogenic Listeria innocua or Escherichia coli K-12. The process was optimized using factorial experimental designs. Optimum process conditions found were 3 cycles at 138°C for 0.1 sec/cycle. Vacuum time was 0.1 sec except for the final vacuum, which was 0.5 sec. Bacterial kill was 1.1–1.4 log cfu/mL. This was comparable to the bacterial kill attained in the previous pilot-plant VSV processor.
The VSV process caused significant mechanical damage to the carcasses in the initial experiments. Some carcasses were ripped in half along the length of the visceral cavity. The main valve appeared to grab the neck area and rip the carcass. They were also ripped on the bottom where the carcass sat on the horizontal section of the mandrel. Many of the carcasses had a leg broken. Modifying the mandrel greatly reduced the mechanical damage, but the legs of most carcasses were still broken.
Although there was still too much mechanical damage, we were scheduled to make the first series of tests at our sister laboratory, the Richard Russell Research Center in Athens, Ga. The center is in the heart of chicken processing country, so we were able to obtain freshly slaughtered carcasses before the chill tank. Since the mobile VSV unit was successful bacteriologically and mechanical damage was greatly diminished, we proceeded with the field tests.
The mobile VSV processor was mounted on a flatbed truck and driven to the center, and experiments were made on two days. The carcasses were obtained at a local plant, placed in an insulated chest, and immediately transported to the center. Travel times were less than 30 min. A 23 factorial design was used on the first day, and 22 on the second. On the first day, the bacterial kill for the naturally occurring E. coli and Campylobacter was 1.2–1.3 log, comparable to the kill found in the pilot plant with L. innocua and E. coli K-12.
The second day showed similar results. E. coli was reduced by 1.0 log. There was little Campylobacter on the control carcasses. Only 4 of 6 controls were positive. However, only 1 of 12 processed carcasses was positive, and that was with only 2 cycles of treatment. None of the samples that were cycled 3 times tested positive.
It was during these field tests that the cause of the leg breakage and mechanical damage became obvious. In the previous pilot-plant processor, a screen was set in the bottom of the treatment chamber to hold the carcass. After the mandrel was added, the screen was considered superfluous. Therefore, in the mobile unit, there was no support screen provided, only the mandrel. The legs of the carcass hung below the chamber when there was no support for the legs. When the main valve closed, one leg got caught and broken. This occurred at the bottom of the valve, so it was not visually obvious during processing. Therefore, when we returned to our laboratory, we added a screen to the mandrel to hold the legs inside the chamber. With the addition of this screen, mechanical damage disappeared.
Bacterial kill was checked in the pilot plant after these adjustments. The bacterial kill was comparable to the previous pilot-plant results and to the field tests. It averaged 1.1–1.6 log for inoculated L. innocua, depending on the source or brand of chicken.
--- PAGE BREAK ---
Application to Other Foods
Although the primary objective of the research was to develop a process to surface-pasteurize chicken carcasses, we also applied the process to various other solid foods.
• Hot Dogs. Hot dogs should be free of Listeria contamination when exiting the cooker. Removal of the casing and atmospheric contamination exposes the product to Listeria after cooking and before packaging. This contamination is on the surface and amenable to surface pasteurization.
In 1998–99, there was a large recall of hot dogs due to contamination with Listeria monocytogenes (CDC, 1998; 1999a, c). Therefore, we applied the VSV process to hot dogs inoculated with L. innocua in the pilot-plant unit and used factorial experimental designs to optimize the process (Kozempel et al., 2000).
Air and moisture on the surface of the hot dog interferes with the rapid energy transfer from the steam to the product. Although the interfering surface layers are removed with the initial vacuum, the condensing steam itself continuously deposits an insulating water (condensate) layer during processing. Cycling between vacuum and steam effectively removed this redeposited water layer and improved surface treatment.
Cycling significantly increased the bacterial kill. At 138ºC, there was a >5-log kill for 2 or 3 cycles. A Cooperative Research and Development Agreement has been signed with an industry partner, Alkar-RapidPak, Inc., Lodi, Wis., to develop a commercial unit integrating the VSV process into RapidPak packaging machines. The agreement is initially aimed at hot dogs, but plans are to expand the project to other ready-to-eat foods. RapidPak intends to introduce a commercial version of the hot dog packaging/pasteurization system in 2004.
• Fruits and Vegetables. There has been an increasing awareness that fruits and vegetables can and do cause foodborne illness. The Centers for Disease Control and Prevention reported that between 1973 and 1987, 2% of the foodborne outbreaks and 2% of the outbreak-associated cases were connected with fresh produce and that between 1988 and 1991 these numbers increased to 5% and 8%, respectively (Tarr et al., 1997). In 1989, 25,000 people were infected by Salmonella from cantaloupes.
We therefore applied the VSV process to various fruits and vegetables (Kozempel et al., 2002). Cantaloupes, grapefruits, and carrots were purchased at the local supermarket and processed as is. The produce was not inoculated. Process conditions were approximated, not optimized, for these initial experiments. Bacterial kill of naturally occurring total aerobic plate count was 3.4 log for cantaloupe, 3.6 log for grapefruit, and >5.0 log for carrots.
We optimized the process for cantaloupes, grapefruits, and beets using factorial experimental designs. Cantaloupes and grapefruits were inoculated. Beets were not inoculated because the naturally occurring bacterial counts were sufficiently high and consistent to make inoculation unnecessary. The bacterial kill ranged from 2.5 log for beets to 3.5 log for cantaloupes and grapefruits. The process conditions were similar to those found with chicken and hot dogs, 0.1–0.2 sec steam time per cycle, 138–143°C steam, and 2 or 3 cycles.
We also applied the process to various other fruits and vegetables using the process conditions established for the previous commodities. Those products that did not have consistently high natural bacterial counts were inoculated with L. innocua. The bacterial kill ranged from 2.5 to 4.7 log.
--- PAGE BREAK ---
Unfortunately, the VSV process did not work for all fruits and vegetables. Peppers exploded. Bananas split and turned black. Broccoli turned bright green, indicative of blanching. The edible part of cauliflower was acceptable, but the stalk and leaves turned bright green, again indicative of blanching. VSV-treated cauliflower would probably be acceptable for fresh-cut products.
• Catfish. Although catfish have not been associated with food poisoning or recalls, we applied the VSV process to it as well (Kozempel et al., 2001b). Douglas Marshall of Mississippi State University shipped eviscerated catfish to our laboratory. After rinsing the catfish, we inoculated it with L. innocua and processed it. We optimized the process using factorial experimental designs. Bacterial kill up to 2.5 log was achieved at optimum process conditions of 4 cycles at 143°C steam, 0.1 sec steam per cycle, and 0.5 sec vacuum time.
Fitting into Existing Processes
For most products, the usual VSV process conditions are 0.1 sec for vacuum and steam, except 0.5 sec for the final vacuum. Steam is saturated at 138 or 143°C, and 2 or 3 cycles are preferred. Under these conditions, there is little or no thermal damage. As shown in Fig. 4, bacterial reduction varies with the product treated. With hot dogs that have a relatively smooth surface, we achieved a 5-log kill with 3 cycles. Fruits and vegetables varied from 2.5- to 4.7-log kill. Chicken carcasses were the hardest to treat. There are the cavity, feather follicles, skin, and meat, plus numerous hiding places for bacteria, such as under the wings and at both ends of the cavity. For chicken, bacterial kill ranged from 1.1 to 1.6 log. The kill was also dependent on the upstream processing operations at the plant and possibly farm conditions, as evidenced by the variation in kill with brand.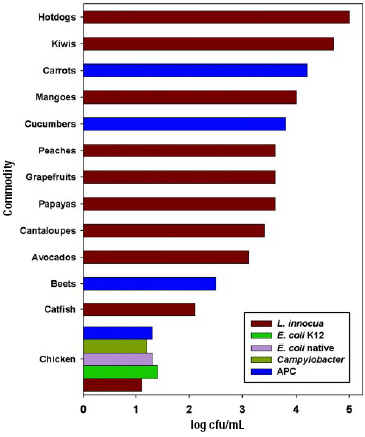
How the VSV process will fit into existing food processes will depend on the product and use. Since the process treatment time is so short, the VSV process can be integrated into existing process lines. The hot dog treatment will be integrated into the packaging step at the last possible moment before sealing. The hot dogs, on a conveyor, will be placed into the plastic package without the top. They will be subjected to the VSV step, then the plastic top will be installed and the package sealed. Although the processing unit would be mechanically quite different from our prototype, the concept would be the same.
The process could be used for fruits and vegetables for the fresh market but would be a better fit for fresh-cut product. For the fresh market, the product would be processed like hot dogs, i.e., processed during packaging. Packaging would prevent recontamination. In a fresh-cut plant, the fruit or vegetable could be processed in the same equipment, without packaging, or in a unit similar to our prototype but with multiple chambers. The VSV process would be used before peeling or cutting to prevent internal contamination.
The VSV process was originally intended for chicken. The process would be inserted into the normal line before the cold water chill tank. An alternative approach would be to use it after the chill tank. Either place would probably use a variation of our prototype with multiple chambers.
Fish will require a different-shape treatment chamber. Either a packaging-type unit or one similar to our prototype with multiple chambers would work. Fish would be filleted after the VSV treatment and the fillets handled as usual.
We have reported on the obvious uses of the technology. There are other not-so-obvious uses that have not been tried or have been tried but not reported yet. For instance, can the process be used to kill horticultural pests on fruits and vegetables? Recent work (as yet unpublished) with lemons infested with red scale insects achieved 100% kill. Can the process kill bacteria on apples? Can it be used on small products like nuts, berries, or seeds? Further research will tell.
Mention of a brand or firm name does not constitute an endorsement by the U.S. Dept. of Agriculture over others of a similar nature not mentioned.
by Michael Kozempel, Neil Goldberg, and James C. Craig Jr.
Authors Kozempel and Goldberg are Engineers, and author Craig is a retired Engineer, with the Eastern Regional Research Service, Agricultural Research Service, U.S. Dept. of Agriculture, 600 E. Mermaid Ln., Wyndmoor, PA 19038. Authors Kozempel and Craig are Professional Members of IFT. Send reprint requests to author Kozempel.
References
Anonymous. 1999. Decontamination issue. Newsletter, Food Refrigeration and Process Engineering Research Centre, Univ. of Bristol, UK.
CDC. 1998. Multistate outbreak of listeriosis–United States. Centers for Disease Control and Prevention, Morbid. Mortal. Wkly. Rept. 47: 1085-1086.
CDC. 1999a. Update: Multistate outbreak of listeriosis–United States, 1998-1999. CDC: Morbid. Mortal. Wkly. Rept. 47: 1117-1118.
CDC. 1999b. Update: Multistate outbreak of listeriosis. Centers for Disease Control and Prevention, Div. of Media Relations. www.cdc.gov/od/oc/media/pressrel/r990114.htm.
Bremer, P.J., Monk, I., Osborne, C.M., Hills, S., and Butler, R. 2002. Development of a steam treatment to eliminate Listeria monocytogenes from King Salmon (Oncorhynchus tshawytscha). J. Food Sci. 67: 2282-2286.
Kozempel, M., Goldberg, N., Radewonuk, E.R., and Scullen, O.J. 2000. Rapid Hot dog surface pasteurization using cycles of vacuum and steam to kill Listeria innocua. J. Food Protect. 63: 457-461.
Kozempel, M., Goldberg, I., Radewonuk, E.R., and Scullen, O.J. 2001a. Modification of the VSV surface pasteurizer to treat the visceral cavity and surfaces of chicken carcasses. J. Food Sci. 66: 954-959.
Kozempel, M., Marshall, D.G., Radewonuk, E.R., Scullen, O.J., Goldberg, N., and Bal’a, M.F. 2001b. A rapid surface intervention process to kill Listeria innocua on catfish using cycles of vacuum and steam. J. Food Sci. 66: 1012-1016.
Kozempel, M., Radewonuk, E.R., Scullen, O.J., and Goldberg, N. 2002. Optimization and application of the vacuum/steam/vacuum surface intervention process to fruits and vegetables. Innov. Food Sci. Emerg. Technol. 3(1): 63-72.
Kozempel, M., Goldberg, N., Dickens, J.A., Ingram, K.D., and Craig, J.C. Jr. 2003. Scale-up and field test of the vacuum/steam/vacuum surface intervention process for poultry. J. Food Proc. Eng. In press.
Morgan, A.I., Goldberg, I., Radewonuk, E.R., and Scullen, O.J. 1996a. Surface pasteurization of raw poultry meat by steam. Lebensm. Wiss u-Technol. 29: 447-451.
Morgan, A.I., Radewonuk, E.R., and Scullen, O.J. 1996b. Ultra high temperature, ultra short time surface pasteurization of meat. J. Food Sci. 61: 1216-1218.
Nutsch, A.L., Phebus, R.K., Riemann, M.J., Schafer, D.E., Boyer, J.E. Jr., Wilson, R.C, Leising, J.D., and Kastner, C.L. 1997.
Phebus, R.K., Nutsch, A.L., Schafer, D.E., Wilson, R.C, Riemann, M.J., Leising, J.D., Kastner, C.L., Wolf, J.R., and Prasai, R.K. 1997. Comparison of steam pasteurization and other methods for reduction of pathogens on surfaces of freshly slaughtered beef. J. Food Protect. 60: 476-484.
Tarr, P.I., Besser, T.E., Hancock, D.D., Keene, W.E., and Goldoft, M. 1997. Verotoxigenic Escherichia coli infection: U.S. overview. J. Food Protect. 60: 1466-1471.
Wilson, R.C., Strong, J., Hocker, J., O’Connor, J. Leising, J. 1999. Apparatus for steam pasteurization of meat. U.S patent 5,976,005.
Wilson, R.C., Leising, J., Strong, J., Hocker, J., O’Connor, J. 2000. Method and apparatus for steam pasteurization of food. U.S patent 6,019,033.
