Nanotechnology in Nutraceuticals and Functional Foods
Emerging technology has shown great potential for delivering bioactive compounds in functional foods to improve human health.
Nanotechnology deals with the capability to image, measure, model, control, and manipulate matter at dimensions of roughly 1–100 nanometers, where novel interfacial phenomena introduce new functionalities. This exceptional capability has led to a vast array of new technologies that have an impact on virtually every aspect of science and technology, industry, economy, the environment, and human lives.
New initiatives have been launched by governments, academia, and private sectors in the United States, Europe, Japan, and most other countries around the globe to ensure rapid development and deployment of nanotechnology to improve the quality of life of all citizens, create jobs, promote economic growth, and enhance the security of our society (Baeumner, 2004; Moraru et al., 2003).
In a visionary presentation, Feynman (1959) suggested that we should be able to manipulate matter down to the atomic level with great control and precision so that novel materials with designed properties could be obtained. However, the real breakthrough that eventually led to the flourishing of the fledgling area in research laboratories did not occur until the mid-1980s, when some key analytical tools such as scanning tunneling microscopy and atomic force microscopy were developed.
The newfound ability to see and move atoms and molecules in a precise manner quickly spread to be utilized in many other fields of sciences. Discovery of new materials such as fullerenes and carbon nanotubes and the characterization of their unique physical and chemical properties were essential in understanding that their properties were governed by quantum mechanics rather than Newtonian mechanics. This opened a whole new world, fostering the imagination and creativity of scientists and engineers. A new wave of scientific discoveries in nanoscale science, engineering, and technology was thus ensured.
Some nanoscale phenomena have been utilized in nutraceutical and functional food formulation, manufacturing, and processes. New concepts based on nanotechnology are being explored to improve product functionality and delivery efficiency (Scott and Chen, 2003; Worldnutra, 2005). Some of these nano-based technologies are outlined below.
Systems containing large interfacial areas such as emulsion, dispersion, and bicontinuous structured fluid are a rich source of new knowledge. Newly developed capabilities in nanoscale characterization offer a better visualization of these structures in nanometer resolution, and further a better understanding of their functionality (Tolles and Rath, 2003).
--- PAGE BREAK ---
Nanoparticulate Delivery Systems
When amphiphilic molecules like surfactants, lipids, and copolymers that have both polar and nonpolar characteristics are dispersed in a polar solvent, hydrophobic interactions cause them to spontaneously self-assemble into a rich array of thermodynamically stable, lyotropic, liquid crystalline phases with characteristic length scales in the nanometers. These include micelles, hexagonal (tubular) structures, lamellar structures, and cubosomes, which possess a high degree of molecular orientation order despite the fact that they exist in a liquid state.
• Micelles are submicron spherical particles, typically 5–100 nm in diameter, that are formed spontaneously upon dissolution of surfactants in water at concentrations that exceed a critical level, known as the “critical micelle concentration” (CMC). This self-assembly process is thermodynamically driven; i.e., interactions of the hydrophobic tail group of surfactants with water are minimized, while interactions of the hydrophilic surfactant head groups with water are maximized. Because of this, micelle integrity under a given set of environmental conditions (pH, temperature, salt concentration) is often maintained for many years.
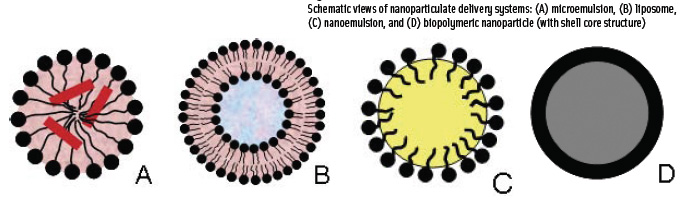 A remarkable property of micelles is that they have the ability to encapsulate nonpolar molecules such as lipids, flavorants, antimicrobials, antioxidants, and vitamins (Weiss and McClements, 2002). Compounds that ordinarily are not water soluble or are only sparingly soluble can, with the help of micelles, be made water soluble. Micelles containing solubilized materials are referred to as microemulsions (Figure 1A) or swollen micelles.
A remarkable property of micelles is that they have the ability to encapsulate nonpolar molecules such as lipids, flavorants, antimicrobials, antioxidants, and vitamins (Weiss and McClements, 2002). Compounds that ordinarily are not water soluble or are only sparingly soluble can, with the help of micelles, be made water soluble. Micelles containing solubilized materials are referred to as microemulsions (Figure 1A) or swollen micelles.
While micelles have been used as a delivery system for pharmaceutical compounds for quite a long time, their use as carrier systems for functional food components has only recently attracted increased attention. Reports of successful application of microemulsions include encapsulation of limonene, lycopene, lutein, and omega-3 fatty acids using a variety of food-grade emulsifiers, although in some cases addition of ethanol as a co-surfactant was required. Patent applications have been filed for the use of microemulsions to incorporate essential oils in flavored carbonated beverages and to encapsulate alpha-tocopherol to reduce lipid oxidation in fish oil (Weiss and McClements, 2002).
• Liposomes, or lipid vesicles, are formed from polar lipids that are available in abundance in nature, mainly phospholipids from soy and egg. Like micelles, liposomes can incorporate a wide variety of functional components in their interior. However, in contrast to micelles, they can be used to encapsulate both water-and lipid-soluble compounds.
Liposomes are spherical, polymolecular aggregates with a bilayer shell configuration (Figure 1B). Depending on the method of preparation, lipid vesicles can be uni- or multi-lamellar, containing one or many bilayer shells, respectively. Liposomes typically vary in size between 20 nm and a few hundred micrometers. Their core is aqueous in nature, its chemical composition corresponding to that of the aqueous solution in which the vesicles are prepared. Because of the charge of the polar lipids used in the preparation of liposomes, charged but water-soluble ionic species can be trapped inside the liposomes. The pH and ionic strength of the liposomal core can thus differ from those of the continuous phase in which the liposomes are later dispersed.
--- PAGE BREAK ---
Liposomes have been successfully used to encapsulate proteins and provide a microenvironment in which proteins can continue to function regardless of external environmental conditions. On the other hand, the interior of the bilayer has properties resembling those of an organic solvent. Consequently, lipid compounds can be encapsulated inside the bilayer, a process known as adsolubilization.
Taylor et al. (2005) reviewed food applications of liposomes. They cited studies on liposomes to increase shelf life of dairy products by encapsulating lactoferrin, a bacteriostatic glycoprotein as well as nisin Z, an antimicrobial polypeptide. Antimicrobial efficiency of other ingredients in the encapsulated form has also been reported (Gaysinksy et al., 2005, Were et al., 2004). Liposomal-entrapped phosvitin was used to inhibit lipid oxidation in a variety of dairy products and ground pork. Liposome-encapsulated vitamin C retained 50% activity after 50 days of refrigerated storage, whereas free ascorbic acid lost all activity after 19 days.
• Nanoemulsions are simply very fine oil-in-water (o/w) emulsions with mean droplet diameter of 50–200 nm (Figure 1C). An emulsion is defined as a mixture of two completely or partially immiscible liquids, such as oil and water, with one liquid being dispersed in the other in the form of droplets. Examples of emulsified food products are mayonnaise, milk, sauces, and salad dressings.
In contrast to these well-known o/w emulsions, nanoemulsions are small enough not to scatter light in the visible region of the spectra; thus, they appear clear instead of being optically opaque. Because of their small size, they also do not cream within an appreciable time. Creaming is the process whereby oil droplets move to the top of the emulsion to form a concentrated oil-droplet layer. This is often followed by a complete breakdown of the emulsion, yielding a clearly visible oil layer on top of the emulsion (McClements and Weiss, 2005).
Nanoemulsions and macro-emulsions can be manufactured in a similar fashion using high-pressure homogenizers, or membrane and microfluidic channels (Nakajima, 2005). It should be noted that the proper choice of surfactants and/or polymers is critical in the production of nanoemulsions.
Because of their small size, nanoparticles have excellent penetration properties to ensure rapid delivery of high concentrations of active ingredients to cell membranes. Bioavailability of lipophilic active ingredients can be substantially improved by delivery in nanoemulsions (Nakajima, 2005). For example, nanoemulsions have been used in parenteral nutrition for quite some time. Also because of their small size, they may also exhibit some interesting textural properties that differ from those of an emulsion containing larger droplets. For example, they may behave like a viscous cream even at low oil-droplet concentrations, a fact that has attracted attention in the development of low-fat products.
--- PAGE BREAK ---
• Biopolymeric nanoparticles consist of a matrix of biopolymers that may be linked through intermolecular attractive forces or through chemical covalent bonds to form solid particles. Nanoparticles may consist of a single biopolymer or may have a core-shell structure (Figure 1D). Because of the versatility in terms of compounds that can be encapsulated and the degree to which these particles can be engineered and surface properties can be tailored, they have rapidly become the most promising nanoscale delivery systems in the pharmaceutical and cosmetics industries.
Polylactic acid, a key component of many biodegradable nanoparticles, was first developed in 1932, but its high cost and susceptibility to hydrolytic breakdown were believed to make it unsuitable for use in biomedical or agricultural applications or sparingly used in research (Lunt, 1998). However, the use of this polymer as an ideal material for sutures was discovered in the 1970s, and a process was developed in the 1980s to produce the polymer via bacterial fermentation, greatly reducing costs and increasing production rates.
Today, a wide variety of natural and synthetic polymers have been used to encapsulate and deliver compounds. Among these are chitosan, a natural antimicrobial and antioxidative polymer obtained from crustacean shells (Shahidi and Abuzaytoun, 2005) and the synthetic polymers L-, D-, and D,L-polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactic acid (PCL). Copolymers created using combinations of the monomers lactide, galactide, and aprolactone are also increasingly used.
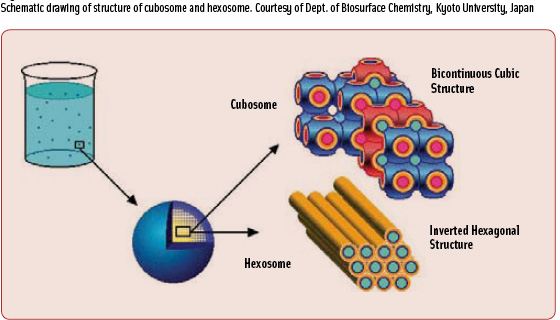 • Cubosomes (Figure 2) are bicontinuous cubic phases which consist of two separate, continuous, but nonintersecting hydrophilic regions divided by a lipid layer that is contorted into a periodic minimal surface with zero average curvature (Spicer, 2004). The continuous and periodic structure results in a very high viscosity of the bulk cubic phase. However, cubosomes prepared in dispersion maintain a nanometer structure identical to that of the bulk cubic phase but yield a much lower, water-like viscosity. Its tortuosity can be useful for slowing diffusion in controlledtransport applications. Its isotropic optical property permits uses in many different products.
• Cubosomes (Figure 2) are bicontinuous cubic phases which consist of two separate, continuous, but nonintersecting hydrophilic regions divided by a lipid layer that is contorted into a periodic minimal surface with zero average curvature (Spicer, 2004). The continuous and periodic structure results in a very high viscosity of the bulk cubic phase. However, cubosomes prepared in dispersion maintain a nanometer structure identical to that of the bulk cubic phase but yield a much lower, water-like viscosity. Its tortuosity can be useful for slowing diffusion in controlledtransport applications. Its isotropic optical property permits uses in many different products.
Compared to liposomes, cubosomes have much higher bilayer area-to-particle volume ratios. The cubosome structure can be changed by modifying the environmental conditions, such as pH, ionic strength, or temperature, thus achieving controlled release of the carried compound.
Cubosomes may be used in controlled release of solubilized bioactives in food matrices as a result of their nanoporous structure (approximately 5–10 nm); their ability to solubilize hydrophobic, hydrophilic, and amphiphilic molecules; and their biodegradability and digestibility by simple enzyme action. The cubic phase is strongly bioadhesive, so it may find applications in flavor release via its mucosal deposition and delivery of effective compounds. Yet, its tortuous structure may lead to applications where masking unpleasant taste or flavor is desirable, because of the slow effective diffusivity. The rate of release appears tunable through system optimization or ideal formulation of products for specific purposes.
--- PAGE BREAK ---
 Production and Applications
Production and Applications
A fairly large number of manufacturing methods are available to produce solid nanoparticles, including nanoprecipitation, solvent evaporation, and spontaneous emulsification followed by solvent diffusion. Several new methods have been developed that use milder chemicals and those that can be easily removed from the final product by such methods as “salting-out” and electrospraying (Figure 3).
Biological methods of producing nanoparticles and nanostructures have also been explored in recent years. One example is using plant viruses as templates to synthesize nanomaterials with predesigned surface functionality for target recognition, controlled gates for substance uptake and release, and imbedded metals and dyes for facilitated imaging (Douglas et al., 2002).
The development of “coated and conducting” DNA molecules has enabled medical researchers to produce a new generation of nanoparticles that combine targeting of cancer cells, controlled release of anticancer drugs, and localized generation of heat inside the cell, thereby dramatically boosting the performance of anticancer drugs. Other applications include the ability to rapidly detect the presence of single molecules (proteins or DNA fragments), eliminating the need for amplification or enrichment. The sensitivity of some of these new techniques is 1,000 times that of conventional polymerase chain reaction (PCR).
Nanotechnology provides tools and means to precisely graft biological and chemical ligands onto the surface of nanoparticles. The surface modification allows nanoparticles to recognize target cells. With a site-specific controlled-release mechanism built in, the nanoparticles can deliver the functional compounds to the target site to increase their effectiveness and efficiency. This capability may allow for better bioavailability and absorption in the gastrointestinal tract.
On the other hand, specific molecular recognition, site targeting, and adhesion action may be used to bind undesirable compounds encountered in the food consumption chain and remove harmful matters from the digestive system of humans and animals. One relevant research discovery is that polystyrene nanoparticles with specifically functionalized adhesion mechanism can bind the nanostructured K88 fimbrial adhesin of Escherichia coli cells in poultry guts and agglomerate them to remove the pathogens prior to slaughtering of birds (Qu et al., 2005). This technology improves food safety by reducing the pathogenic load. The safety of using such nanoparticles has been preliminarily demonstrated through an array of in-vitro and in-vivo biosafety tests. This is an important step to assure a responsible and safe deployment of this new nanotechnology.
For the recognition and binding of specific molecules and cellular structures, molecular imprinting techniques are another set of tools in which a variety of polymers are created that can form complementary nanoscale binding pockets for the intended targets. Development of new and creative approaches to identify nanoscale binding domains with complementary ligands for selective recognition in food systems will help to accelerate the development of intelligent biomolecule–modulated protein and bioactive-compound delivery systems.
--- PAGE BREAK ---
Targeted delivery systems have greater benefits and fewer adverse effects. For example, one can imagine that targeted delivery of a salty taste will require much less salt in the bulk of foods to achieve the desirable salty sensation. Reduced salt intake will help improve sodium ion–induced chronic conditions such as hypertension and heart disease.
Molecular or cellular specific-targeted delivery systems may find applications in individualized food consumption to ensure optimal health. For example, one may envision that in the future the recognition capability will allow carrying a bioactive compound to be only released in the presence of certain critical biochemical or genetic markers that are specific to each individual consumer.
Ultimately, advances in nanotechnology may lead to multifunctional nanoscale nutraceutical delivery systems that can simultaneously detect and recognize the appropriate location, analyze the local and global needs, decide whether or how much of the payload should be released, and monitor the response for feedback control. This is in analogy to “smart drug delivery” or “intelligent therapeutics” currently under investigation in the medical nanotechnology field.
Great Potential
Thus, nanotechnology has shown great potential for improving the effectiveness and efficiency of delivery of nutraceuticals and bioactive compounds in functional foods to improve human health. It can enhance solubility, facilitate controlled release, improve bioavailability, and protect the stability of micronutrients and bioactive compounds during processing, storage, and distribution. It can also lead to the development of new flavor delivery systems to improve food quality and functionality. Controlled release may eventually lead to in-situ flavor and color modification of products.
Ultimately, understanding the mechanism of targeted delivery will provide a foundation that will enable food manufacturers to design smart food systems capable of ensuring the optimal health of each individual citizen.
Defining Nanotechnology
Definitions of nanotechnology vary greatly, and it is imperative to establish a consistent definition.
The U.S. National Nanotechnology Initiative (NSTC, 2005b) has defined “nanotechnology” as encompassing the science, engineering, and technology related to the understanding and control of matter at approximately 1–100 nm. Furthermore, it emphasizes nanotechnology research and development of materials, devices, and systems with novel properties and functions due to their nanoscale dimensions or components, and stresses the ability to measure/control/manipulate matter at this length scale to change those properties and functions and integrate along length scales upward for many fields of applications.
--- PAGE BREAK ---
It has been rather challenging to distinguish research and development nanotechnology from some types of ongoing scientific research that have achieved a certain level of miniaturization or that operate at a nanometer-length scale, especially at the intersection of nanotechnology and biology, where many biological structures and processes regularly occur on the nanoscale, such as DNA and enzymes. The National Institutes of Health offers the following corollary (NSTC, 2005a):
“While much of biology is grounded in nanoscale phenomena, NIH has not re-classified most of its basic research portfolio as nanotechnology. Only those studies that use nanotechnology tools and concepts to study biology; that propose to engineer biological molecules toward functions very different from those they have in nature; or that manipulate biological systems by methods more precise than can be done by using molecular biological, synthetic chemical, or biochemical approaches that have been used for years in the biology research community are classified as nanotechnology projects.”
Since agriculture and food science are mostly involved in biological systems, it is advisable to embrace this definition, which focuses on the novel properties that occur at the nanoscale and makes a distinction between nanotechnology and biology.
Hongda Chen is National Program Leader for Bioprocess Engineering and Nanotechnology, Cooperative State Research, Education and Extension Service, U.S. Dept. of Agriculture, Washington, DC 20250-2220 ([email protected]). Jochen Weiss is Assistant Professor, Dept. of Food Science, University of Massachusetts, Amherst, MA 01003 ([email protected]). Fereidoon Shahidi, a Fellow of IFT, is University Research Professor, Dept. of Biochemistry, Memorial University of Newfoundland, St. John’s, NL, A1B 3X9, Canada ([email protected]). The authors are Professional Members of IFT.
References
Baeumner, A. 2004. Nanosensors identify pathogens in food. Food Technol. 58(8): 51-55.
Douglas, T., Allen, M., and Young, M. 2002. Self-assembling protein cage systems and applications in nanotechnology. In “Biopolymer,” Vol. 8, ed. A. Steinbuched, pp. 405-426. Wiley-VCH, Weinheim.
Feynman, R.P. 1959. There is plenty of room at the bottom. Presented at Ann. Mtg., Am. Physical Soc., California Inst. of Technology, Dec. 29.
Gaysinksy, S., Davidson, P.M., Bruce, B.D., and Weiss, J. 2005. Stability and antimicrobial efficiency of eugenol encapsulated in surfactant micelles as affected by temperature and pH. J. Food Protect. 68: 1359-1366.
Lunt, J. 1998. Large-scale production, properties and commercial applications of polylactic acid polymers. Polymer Degradation Stability 59: 145-152.
McClements, D.J. and Weiss. J. 2005. Lipid emulsions. In “Bailey’s Industrial Oil and Fat Products,” Vol. 3, ed. F. Shahidi, pp. 457-502. Wiley-Interscience, New York.
Moraru, C.I., Panchapakesan, C.P., Huang, Q., Takhistove, P., Liu, S., and Kokini, J.L. 2003. Nanotechnology: A new frontier in food science. Food Technol. 57(12): 24-29.
Nakajima, M. 2005. “Development of Nanotechnology and Materials for Innovative Utilization of Biological Functions.” Proceedings of the 34th United States and Japan Natural Resources (UJNR) Food and Agriculture Panel, Susono, Japan.
NSTC. 2005a. The National Nanotechnology Initiative: Research and development leading to a revolution in technology and industry—Supplement to the President’s 2006 budget. Nanoscale Science, Engineering, and Technology (NSET) Subcommittee, National Science and Technology Council, U.S. Govt., Washington, D.C., March.
NSTC. 2005b. The National Nanotechnology Initiative: Strategic plan. Nanoscale Science, Engineering, and Technology (NSET) Subcommittee, National Science and Technology Council, U.S. Govt., Washington, D.C., December.
Qu, L., Luo, P.G., Taylor, S., Lin, Y., Huang, W., Tzeng, T.-R.J., Stutzenberger, F., Latour, R.A., and Sun, Y-P. 2005. Visualizing adhesion-induced agglutination of Escherichia coli with mannosylated nanoparticles. J. Nanosci. Nanotechnol. 5: 319-322.
Scott, N. and Chen, H. 2003. Nanoscale science and engineering for agriculture and food systems, Report submitted to Cooperative State Research, Education and Extension Service (CSREES), U.S. Dept. of Agriculture. www.nseafs.cornell.edu.
Shahidi, F. and Abuzaytoun, R. 2005. Chitin, chitosan and co-products: Chemistry, production, applications and health effects. Adv. Food. Nutr. Res. 49: 93-137.
Spicer, P. 2004. Cubosomes: Bicontinuous cubic liquid crystalline nanostructured particles. In “Encyclopaedia of Nanoscience and Nanotechnology,” ed. J.A. Schwarz, C. Contescu, and K. Putyera. Marcel Dekker, New York.
Taylor, T.M., Davidson, P.M., Bruce, B.D., and Weiss, J. 2005. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 45: 1-19.
Tolles, W.M. and Rath, B.B. 2003. Nanotechnology, A stimulus for innovation. Curr. Sci. 85: 1746-1759.
Weiss, J. and McClements, D.J. 2002. Mass transport phenomena in emulsions containing surfactants. In “Encyclopedia of Surface and Colloid Science,” ed. P. Somasundaran and A. Hubbard, pp. 3123-3151. Marcel Dekker, New York.
Were, L.M., Bruce, B.D., Davidson, P.M., and Weiss, J. 2004. Size, stability and entrapment efficiency of phospholipid nanocapsules containing polypeptide antimicrobials. J. Agric. Food Chem. 51: 8073-8079.
Worldnutra. 2005. 6th International Conference and Exhibition on Nutraceuticals and Functional Foods. Anaheim, Calif., Oct. 16-19. www.worldnutra.com.
