DNA-Based Subtyping Methods Facilitate Identification of Foodborne Pathogens
Advances in subtyping methods enhance foodborne disease surveillance, but there are challenges in integrating subtyping information with epidemiological data to quickly find the food culprit in an outbreak.
Microbial pathogens have been estimated, in a study published in 1999, to cause about 76 million cases of foodborne illnesses annually in the United States, including an estimated 325,000 and 5,000 cases that result in hospitalizations and deaths, respectively (Mead et al., 1999). A better understanding of foodborne disease transmission, including accurate estimates of attribution of foodborne illnesses to sources, are sorely needed to improve our ability to better control and prevent foodborne illnesses. In addition, rapid detection of foodborne disease outbreaks and their sources is needed to limit the scope of outbreaks and thus the burden of foodborne disease.
Methods to differentiate pathogens beyond the species level (i.e., subtyping methods) represent a critical tool that can facilitate improved source attribution as well as improved foodborne disease surveillance. Furthermore, subtyping methods can be critical in identifying foodborne pathogen transmission from farm-to-table, including identification and characterization of in-plant contamination sources and pathogen persistence.
Classical Subtyping Methods
Classical and phenotypic methods to subtype foodborne pathogens (e.g., serotyping, phage typing) have been used for more than 50 years by public health agencies around the world to aid in foodborne disease surveillance. Serotyping represents the classical phenotypic subtyping method that is probably most familiar to food scientists. Serotyping involves characterization of microbial surface molecules through use of antibodies and antisera. While serotyping has been used for characterization of a variety of foodborne pathogens, serotype characterization of Salmonella isolates represents one of the most important applications of phenotypic subtyping in food safety. More than 2,500 Salmonella serotypes are known; common serotypes relevant to food safety include Typhimurium, Enteritidis, Dublin, and Newport.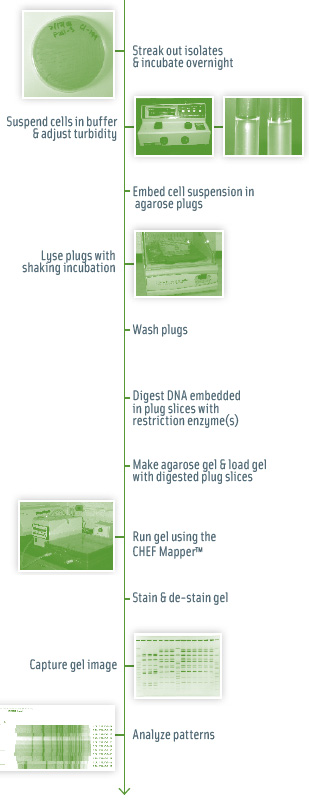
Salmonella serotypes provide a good example of the potential uses of subtyping in food safety. For example, some Salmonella serotypes show host adaptation, meaning that they predominantly affect a specific animal host, even though they can also cause disease in other hosts. Associations between specific serotypes and host species (e.g., Salmonella Enteritidis and poultry; Salmonella Dublin and cattle) can aid in attribution of human Salmonella cases to primary reservoir species. For example, most human salmonellosis cases caused by Salmonella Enteritidis can be attributed to poultry, even though the specific route of transmission could range from consumption of undercooked poultry or eggs to contamination of vegetables (possibly due to waste runoff from poultry farms or use of untreated poultry litter in primary production). Salmonella serotype data (in conjunction with some phage typing data) have been used for source attribution efforts in Denmark, providing estimates on the contributions of Salmonella originating from different host species to the burden of human disease (Hald et al., 2004). In addition, serotype characterization of Salmonella isolates can also aid in detection of salmonellosis outbreaks. While detection of outbreaks caused by common Salmonella serotypes (e.g., Typhimurium) can rarely be accomplished by serotyping alone (as a large number of sporadic cases caused by a common serotype occur in a given time period), outbreaks caused by rare Salmonella serotypes can often be identified based on serotype data. For example, a marked increase in the number of salmonellosis cases in France caused by Salmonella Montevideo, a serotype rarely associated with human disease, facilitated the initial detection of a 2006 salmonellosis outbreak in France (CDC, 2007).
--- PAGE BREAK ---
Molecular Subtyping Methods
While classical and phenotypic subtyping methods for foodborne pathogens have been critical for facilitating foodborne disease surveillance over many decades, there are a number of shortcomings that are often associated with these methods, including, but not limited to, (1) limited discriminatory power, (2) limited standardization and reproducibility, and (3) need for standardized reagents that can be difficult to replace (e.g., antisera). Molecular and, specifically, DNA-based subtyping methods have been developed to overcome many of the shortcomings of classical and phenotypic subtyping methods. Molecular methods, in particular, often provide increased discriminatory power over classical phenotypic methods.
Many of the initial DNA-based subtyping methods developed for foodborne pathogens relied on the generation of “banding patterns” from either (1) genomic and/or plasmid DNA, typically through restriction digestion, or from (2) DNA fragments that were amplified by polymerase chain reaction (PCR). A vast number of different banding pattern-based subtyping methods have been applied to foodborne pathogens and have been detailed in comprehensive review articles (e.g., Wiedmann et al., 2002; Liu et al., 2006). PCR-based subtyping methods that generate banding patterns include Amplified Fragment Length Polymorphisms (AFLP) as well as a number of methods that rely on PCR-based generation of multiple DNA fragments, such as Random-Amplified Polymorphic DNA (RAPD) and Repetitive Element (REP) PCR. A commercial platform for REP-PCR, DiversiLabTM system from bioMérieux Industry (Marcy l'Etoile, France) has recently become available and specific kits for subtyping of major foodborne pathogens (e.g., Campylobacter, Salmonella, Escherichia coli, Listeria) and spoilage organisms (e.g., Acinetobacter, Lactobacillus, Pseudomonas) are also available.
The two banding pattern-based subtyping methods that have been most widely used to characterize foodborne pathogens include (1) ribotyping and (2) Pulse Field Gel Electrophoresis (PFGE). Both of these methods include generation of DNA fragments via restriction enzymes, which cut DNA at a specific target sequence (e.g., the restriction enzyme XbaI specifically cuts DNA when the sequence TCTAGA occurs). In ribotyping, total bacterial DNA is digested by a frequent cutting restriction enzyme, generating a large number of mainly fairly small DNA fragments, which are separated by size through agarose gel electrophoresis. A subsequent Southern blot step uses DNA probes to specifically label and detect those DNA fragments that contain the bacterial genes encoding the ribosomal RNA (rRNA), yielding DNA banding patterns based on only those DNA fragments that contain the rRNA genes. A completely automated, standardized system for ribotyping (RiboPrinter® Microbial Characterization system) is commercially available from DuPont Qualicon (Wilmington, Del.) and has been used to perform molecular subtyping of a number of foodborne pathogens. While typically less discriminatory than PFGE, automated ribotyping provides for rapid turnaround subtyping with limited hands-on labor.
In PFGE, whole bacterial DNA (e.g., chromosomal and plasmid DNA) is cut by a rare cutting restriction enzyme. PFGE typing of a given isolate can be performed using different restriction enzymes in separate reactions to achieve improved discrimination. Restriction enzymes are chosen to cut DNA into approximately 8 to 25 DNA fragments, ranging from approximately 20 kB to >1 Mb (Hunter et al., 2005). Since DNA fragments this large cannot be separated by standard gel electrophoresis techniques, a specific electrophoresis technique using alternating electric fields (i.e., “pulsed fields”) is used for size separation of the resulting DNA fragments, which will subsequently be visualized as DNA banding patterns (Fig. 1). DNA banding patterns for different bacterial isolates are compared, typically using image analysis and bioinformatics software, to differentiate distinct bacterial subtypes (i.e., those with different banding patterns) from those that share identical (or very similar) DNA fragment patterns (Fig. 2).
Often, different restriction enzymes are used for PFGE typing of different foodborne pathogens. For example, while the U.S. Centers for Disease Control and Prevention (CDC) uses the restriction enzymes AscI and ApaI for PFGE typing of L. monocytogenes, the restriction enzymes XbaI and BlnI (AvrII) are typically used for PFGE typing of Salmonella and Escherichia coli O157:H7 isolates. PFGE is probably the most well known subtyping method for foodborne pathogens. The CDC and state health departments in the U.S. have developed a national network (PulseNet) to rapidly exchange standardized PFGE subtype data for isolates of foodborne pathogens, including food isolates contributed by U.S. Food and Drug Administration (FDA) and U.S. Department of Agriculture-Food Safety Inspection Service (USDA-FSIS) laboratories (Gerner-Smidt et al., 2006) (Fig. 3a). PulseNet also is being expanded into an international subtype network (Swaminathan et al., 2006), which has and will continue to facilitate detection of multi-country foodborne disease outbreaks (Fig. 3b). 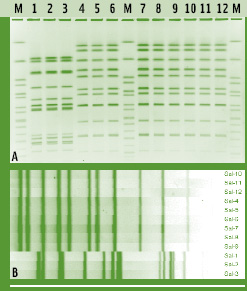
While PFGE subtyping shows a high level of discrimination for many foodborne bacterial pathogens and thus is often considered the current gold standard for discriminatory ability, PFGE does not always have adequate discriminatory power for certain pathogen subtypes (e.g., certain Salmonella Typhimurium, E. coli O157:H7, and L. monocytogenes strains). For example, it has been shown that two unrelated listeriosis outbreaks in different continents have been caused by the same L. monocytogenes PFGE type, which was also found in farm environments unrelated to these outbreaks (Fugett et al., 2007). Hence, despite the wide adoption of PFGE for subtyping of foodborne pathogens, additional, more discriminatory subtyping methods are needed and are being developed. 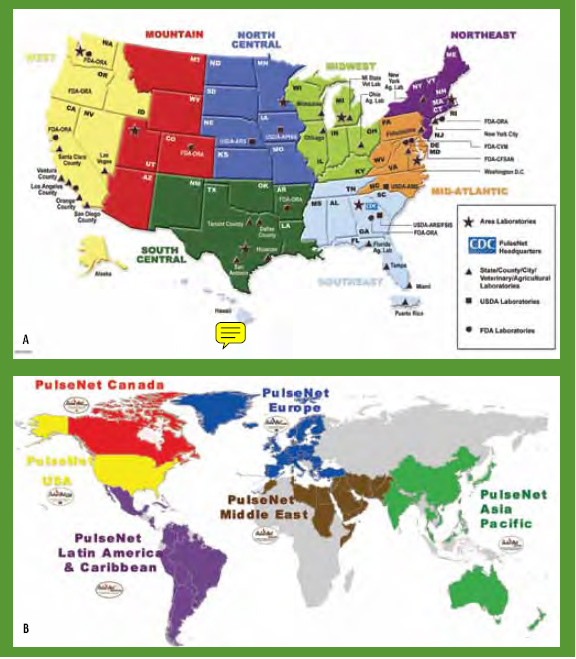
--- PAGE BREAK ---
DNA Sequence-based Subtyping Methods
With the increased availability of DNA sequence and full genome sequencing data for foodborne pathogens, a number of different DNA sequencing-based subtyping methods for foodborne pathogens have been developed. While the underlying genetic changes responsible for differences in banding pattern-based subtypes among isolates are typically not known, DNA sequence-based subtyping methods provide an opportunity to identify the actual genetic changes that differentiate pathogen isolates and thus allow for inference of evolutionary relationships among strains. DNA sequencing-based subtyping methods also typically provide data that are more reproducible as compared to banding pattern-based subtyping methods and that can be easily exchanged, using Internet-based tools and databases.
The most commonly used DNA sequencing-based subtyping methods are multilocus sequence typing (MLST) and multilocus variable number tandem repeat analysis (MLVA). MLST involves sequencing of small fragments (typically 400–700 nucleotides long) of multiple, typically 7, genes in different locations in a bacterial genome (Maiden, 2006). While MLST has been used in a number of studies on the evolution of bacteria, it has only been rarely used in foodborne disease surveillance and outbreak detection, predominantly because it is typically less discriminatory than PFGE (e.g., Torpdahl et al., 2005). On the other hand, sequencing of one or more complete or partial viral genes is used for subtyping of foodborne viral pathogens, including noroviruses and has made critical contributions to surveillance of viral diseases (Siebenga et al., 2008; Shinkawa et al., 2009).
MLVA is a PCR-based method that subtypes organisms by determining the number of a variable number of tandem repeats (VNTR) found in multiple regions in the bacterial genome (Fig. 4). This method allows for very sensitive subtype discrimination. It has been well documented that short sequence DNA repeats and specifically VNTRs can rapidly change in bacterial genomes (e.g., by addition or deletion of one or more repeats). While MLVA has been shown to allow for subtype discrimination beyond that achieved by PFGE in a number of pathogens (e.g., Bacillus anthracis, Salmonella Typhimurium), further efforts on standardization of MLVA and improvements in the ability to compare MLVA patterns generated on different analysis platforms are needed to facilitate use of this technique in typing networks such as PulseNet. MLVA has been applied to foodborne disease surveillance and outbreak detection (e.g., Salmonella Typhimurium) (Torpdahl et al., 2006) and has been used to confirm PFGE findings in the 2009 salmonellosis outbreak linked to peanut butter contaminated with Salmonella Typhimurium (http://www.cdc.gov/salmonella/typhimurium/strains_table.html).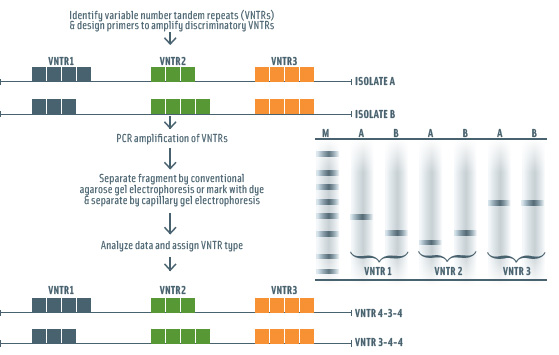
While the full genomes of most bacterial foodborne pathogens range from approximately 3 to 6 million nucleotides, sequencing of bacterial genomes can now be achieved in a few days and at costs of $5,000 to $15,000 (depending on the methodology used) per unfinished genome, which can provide valuable subtype information. Even though bioinformatics analyses of genome sequences may add additional time and costs, full genome sequencing is rapidly becoming a possible method for subtype identification of bacterial isolates, particularly as costs are likely to continue to decline. In fact, full genome sequence data for food and human isolates from one human listeriosis case in 1988 and one outbreak in 2000, both linked to the same processing facility, were used to clarify the relationship between these incidences and suggested that a specific L. monocytogenes subtype persisted in this facility for 12 years with very limited genetic changes (Orsi et al., 2008). In addition, full genome sequencing of foodborne pathogen isolates also allows for identification of appropriate genetic targets that can be used for other DNA-based subtyping methods, e.g., MLST, MLVA, and subtyping methods that characterize single nucleotide changes (e.g., a mutation from A to T) in target genomes (i.e., SNP [single nucleotide polymorphism]-based subtyping methods).
Future Challenges and Opportunities
While subtyping methods, and particular DNA-based subtyping methods, have had tremendous positive impacts on both foodborne disease surveillance and our understanding of foodborne pathogens transmission, ecology, and evolution, a number of challenges remain as researchers and public health agencies worldwide try to fully capitalize on the methodological advances in molecular subtyping of foodborne pathogens. Many of the challenges are related to the fact that subtyping data must be used in conjunction with time- and labor-intensive epidemiological investigations in order to allow for precise definition of foodborne disease outbreaks and, most importantly, to allow for rapid identification of a food vehicle responsible for a foodborne disease outbreak and subsequent removal of that food product from market.
--- PAGE BREAK ---
To illustrate, PFGE (as well as other subtyping methods) may sometimes detect small genetic differences (which, for example, can yield differences in two to three bands in PFGE patterns) between isolates as bacteria can change very rapidly (e.g., during infection in a human or during culturing in the laboratory). Hence, a bacterial isolate from a food may be found to be different from a bacterial isolate from a human clinical case by molecular subtyping, despite the fact that the pathogen present in the food was responsible for the patient’s illness. On the other hand, the detection of an identical PFGE type (or a subtype determined by another method) in two samples (e.g., a food sample and a sample from a clinically affected human) does not necessarily imply a causal relationship or a link between these two isolates, particularly if that subtype is commonly isolated along the food chain. Rather, in outbreak investigations, molecular subtyping information needs to be analyzed in conjunction with epidemiological data to determine causal relationships between the presence of a bacterial strain in food and human disease. In particular, there is a need to develop appropriate methodologies to integrate already quantitative epidemiological data (i.e., odds ratios and their confidence intervals) with measures that quantitatively assess subtype data matches and similarities, in order to allow for a final single quantitative estimate of the “molecular epidemiology” findings (e.g., % likelihood that a given source is responsible for an outbreak given a subtype match and certain odds ratio from the epidemiological investigation).
It is also likely that increased use of molecular subtyping methods leads to improved detection of foodborne disease clusters and outbreaks and may actually contribute to a perceived increase in the number of reported foodborne disease outbreaks. As more disease clusters and outbreaks are detected, the need for epidemiological follow-up investigations increases, even to a point where some state and federal agencies have insufficient resources to rapidly and thoroughly investigate all foodborne disease clusters detected by subtyping. This is compounded by the fact that while molecular subtyping methods have become increasingly rapid and automated, epidemiological investigations continue to require considerable time and human resources. Hence, one of the main specific challenges in the application of molecular subtyping methods is the development of resources to allow for appropriate epidemiological investigations of potential disease clusters identified by molecular subtyping. For example, it is likely that more rapid in-depth epidemiological investigations of relevant subtype data would have facilitated earlier identification of the food vehicle responsible for the U.S. E. coli O157:H7 outbreak linked to spinach in 2006 (Jay et al., 2007) or the sources of other E. coli O157:H7 outbreaks.
Martin Wiedmann, Ph.D., a Professional Member of IFT, is Associate Professor, Dept. of Food Science, Cornell University, Ithaca, N.Y. 14853 ([email protected]). Kendra Nightingale, Ph.D., a Member of IFT, is Assistant Professor, Dept. of Animal Sciences, Colorado State University, Fort Collins, Colo. 80523 ([email protected]).
Acknowledgements
The authors thank Yesim Soyer and Shanna Williams for assistance with preparation of figures and Peter Gerner-Smidt for providing illustrations for national and international PulseNet foodborne disease surveillance networks. Work on molecular subtyping in the author’s laboratories is supported by USDA Special Research Grants (to MW) and National Integrated Food Safety Initiative Grants (Special Emphasis Grant No. 2005-51110-03278 and Grant No. 2008-51110-04333) of the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture. Opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the U.S. Department of Agriculture.
Molecular Subtyping Methods Symposium and Workshop
In addition to their research on molecular subtyping methods and molecular epidemiology, the authors Nightingale (Colorado State University) and Wiedmann (Cornell University) are committed to providing training for current and future professionals in the food industry, government agencies, and academic institutions in the areas of molecular detection and subtyping of food-related microorganisms. The authors have organized “Molecular Methods in Food Microbiology Symposium and Workshop Series: Molecular Subtyping of Food Associated Microorganisms,” August 3–7, 2009, Colorado State University, Fort Collins, Colo.
This new workshop series is partially supported by a USDA National Integrated Food Safety Initiative grant awarded to Nightingale and Wiedmann. To develop the workshop, Nightingale and Wiedmann partnered with Mark Carter and Sarita Raengpradub from Silliker Food Science Center and assembled an advisory board comprised of food industry and regulatory agency scientists. The 2nd annual workshop will focus on DNA subtyping and characterization of food-associated microorganisms.
Program topics will include, but will not be limited to, DNA banding pattern-based subtyping methods (e.g., ribotyping, pulsed field gel electrophoresis, REP-PCR), and typing and characterization methods based on 16S rDNA sequencing. Principles of molecular epidemiology and their application to foodborne disease outbreak investigations will also be covered.
For detailed information on the workshop schedule and registration instructions, please visit the workshop page at http://ansci.colostate.edu/content/view/734/107/.
References
Dominguez, M., Jourdan-Da Silva, N., Vaillant, V., Pihier, N., Kermin, C., Weill, F.X., Delmas, G., Kerouanton, A., Brisabois, A., and de Valk, H. 2008. Outbreak of Salmonella enterica serotype Montevideo infections in France linked to consumption of cheese made from raw milk. Foodborne Pathog Dis. 6: 121-128.
Fugett, E.B., Schoonmaker-Bopp, D., Dumas, N.B., Corby, J., and Wiedmann, M. 2007. Pulsed field gel electrophoresis (PFGE) analysis of temporally matched Listeria monocytogenes isolates from human clinical cases, foods, ruminant farms, and urban and natural environments reveals source associated as well as widely distributed PFGE types. J. Clin. Microbiol. 45: 865-873.
Gerner-Smidt, P., Hise, K., Kincaid, J., Hunter, S., Rolando, S., Hyytia-Trees, E., Ribot, E. M., Swaminathan, B., and Pulsenet Taskforce. PulseNet USA: a five-year update. Foodborne Pathog. Dis. 3: 9-19.
Hald, T., Vose, D., Wegener, H.C., and Koupeev, T. 2004. A Bayesian approach to quantify the contribution of animal-food sources to human salmonellosis. Risk Anal. 24: 255-269.
Hunter, S.B., Vauterin, P., Lambert-Fair, M.A., Van Duyne, M.S., Kubota, K., Graves, L., Wrigley, D., Barrett, T., and Ribot, E. 2005. Establishment of a universal size strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43: 1045-1050.
Jay, M .T., Cooley, M., Carychao, D., Wiscomb, G.W., Sweitzer, R.A., Crawford-Miksza, L., Farrar, J.A., Lau, D.K., O'Connell, J., Millington, A., Asmundson, R.V., Atwill, E.R., and Mandrell, R.E. 2007. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 13: 1908-1911.
Liu, D. 2006. Identification, subtyping and virulence determination of Listeria monocytogenes, an important foodborne pathogen. J. Med. Microbiol. 55: 645-659. Maiden, M.C. 2006. Multilocus sequence typing of bacteria. Ann. Rev. Microbiol. 60: 561-588.
Orsi, R.H., Borowsky, M., Lauer, P., Young, S.K., Nusbaum, C., Galagan, J.E., Birren, B.W., Ivy, R.A., Sun, Q., Graves, L.M., Swaminathan, B., and Wiedmann, M. 2008. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genomics 9: 539.
Siebenga, J., Kroneman, A., Vennema, H., Duizer, E., and Koopmans, M. 2008. Food-borne viruses in Europe network report: the norovirus GII.6 2006B (for US named Minerva-like, for Japan KobeO34-like, for UK V6) variant now dominant in early seasonal surveillance. EURO Surveill. 13: 8009.
Shinkawa, N., Noda, M., Yoshizumi, S., Tokutake, Y., Shiraishi, T., Arita-Nishida, T., Nishio, O., Oka, T., Hansman, G.S., Takeda, N., and Kimura H. 2009. Molecular epidemiology of noroviruses detected in food handler-associated outbreaks of gastroenteritis in Japan. Intervirology. 51: 422-426.
Swaminathan, B., Gerner-Smidt, P., Ng, L.K., Lukinmaa, S., Kam, K.M., Rolando, S., Gutierrez, E.P., and Binsztein, N. 2006. Building PulseNet International: an interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disease outbreaks and emerging foodborne diseases. Foodborne Pathog. Dis. 3: 36-50.
Torpdahl, M., Skov, M.N., Sandvang. D., and Baggesen, D.L. 2005. Geno-typic characterization of Salmonella by multilocus sequence typing, pulsed-field gel electrophoresis and amplified fragment length polymorphism. J. Microbiol. Methods. 63: 173-184.
Torpdahl, M., Sorensen, G., Ethelberg, S., Sando, G., Gammelgard, K., and Porsbo, L.J. 2006. A regional outbreak of S. Typhimurium in Denmark and identification of the source using MLVA typing. Euro Surveill. 11: 134-136.
Wiedmann, M. 2002. Molecular subtyping methods for Listeria monocytogenes. J. AOAC Int. 85: 524-531.
