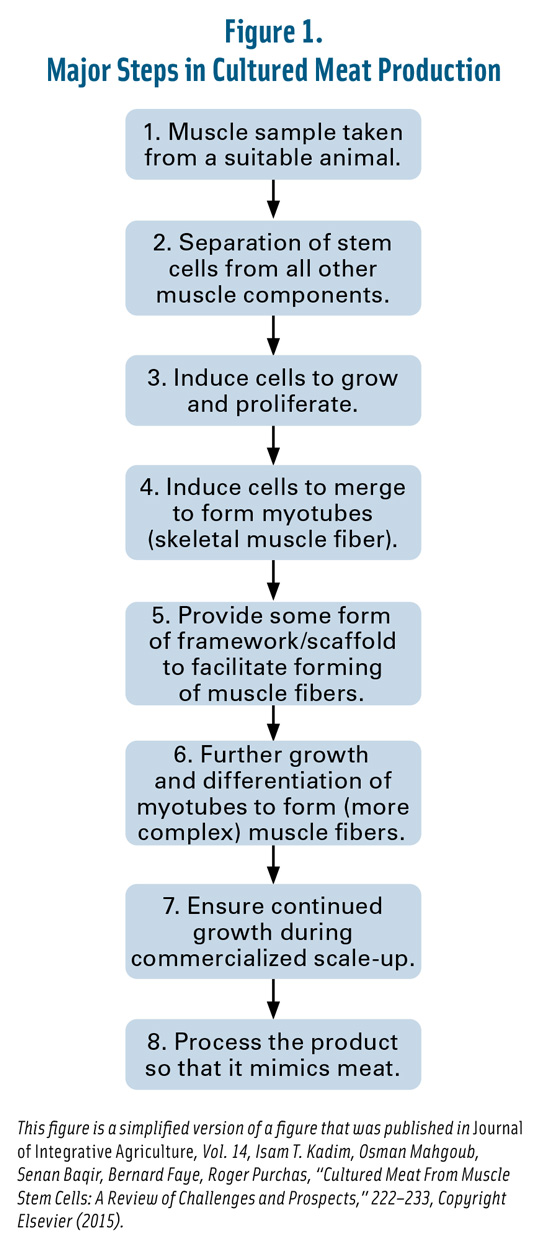
Are We Ready for Cultured Meat?
FOOD SAFETY AND QUALITY
Affordable and sustainable high-protein alternatives are needed for a growing global population whose meat consumption is expected to double from 1999 to 2050 (FAO 2006). With livestock production responsible for over 18% of global greenhouse gas emissions, as well as being one of the major causes of deforestation, wildlife habitat degradation, and eutrophication of water (FAO 2006), could growing muscle tissue in a laboratory or production facility be one potential solution?
Cultured meat, also called lab-grown or in vitro meat, is a controversial technology. Although tissue engineering of muscle and fat is relatively well-understood and performed at the laboratory level (Allan et al. 2019), production of cultured meat for human or pet consumption is still under development. The peer-reviewed literature for cultured meat technology consists mostly of review articles rather than process improvements or technological innovations, and startups, not academics, are the drivers behind technical advances and upscaling (Allan et al. 2019).
Creating Cultured Meat
Muscle stem cells from live animal donors have received the most scrutiny as prospective raw materials for cultured meat. The first step in growing cultured meat from these cells involves a biopsy from a living or recently slaughtered animal, which leads to many questions: Who will raise these animals and under what conditions? Will we now have donor factories with animals raised in confinement to provide muscle cells while enduring short-term pain? What happens to the animals when they are no longer needed as donors? Will cultured cells from slaughtered animals provide another profit stream for the meat industry?
Scientists in the Netherlands have already addressed many of these questions. They describe cultured meat as having the potential to be more resource efficient, sustainable, and animal friendly, reducing the number of cattle worldwide by many orders of magnitude. They estimated that one biopsy from one donor animal could replace the slaughter of 20 cattle if a multiplicity factor of 107 was used, i.e., 5,000 kg of beef from a 500 mg biopsy (Melzener et al. 2020).
The optimal bovine cell donor is a young bull kept in extensive holding conditions and fed a roughage-based diet (Melzener et al. 2020). The biopsy would be taken from the chuck muscle while the animal was still only a few months old, to optimize growth and efficiency of the cell culture. A small incision (15 g) was recommended over the needle biopsy (0.5 g) due to the larger size obtainable.
The animal would need to be immobilized in a treatment cage and sedated with xylazine, and local anesthesia would be administered to minimize pain and ensure the safety of the veterinarian performing the procedure. Because the operation is stressful, many biopsies would be taken at once to optimize the procedure. It would be possible to take repeated biopsies from different positions after a 3-week interval. Studies on mammary gland biopsies revealed full healing after 7 days, with liver biopsies showing no pain-associated behaviors at 20 hr postprocedure (Melzener et al. 2020).
The ultimate fate of the donor calves would be the same as that of traditionally raised cattle: slaughter. To let the donor animals live out their lives and die from natural causes would be inefficient from an environmental perspective (Melzener et al. 2020), and traditional cuts of meat, hide, and offal could also be obtained from the slaughtered donor. To avoid the donor situation, stem cells could be taken from the tissue of freshly slaughtered animals, perhaps providing a new market for the meat industry and potentially offsetting some income loss from fewer animals being slaughtered. Whether this would be acceptable to consumers who want to prevent animals from being slaughtered is an open question, though, since the majority of Western consumers consider the moral promise for animals the most appealing feature of cultured meat (Sharma et al. 2015).
Cell Culture Inputs
Cell culture media have been developed and used extensively for medical and research purposes (Allan et al. 2019), but these media require inputs from animal sources. Fetal calf serum, horse serum (Allan et al. 2019), growth factors, or hormones from genetically engineered E. coli and cyanobacteria hydrolysate (Tuomisto and de Mattos 2011), just to name a few, are required to ensure cell survival, growth, and proliferation.
Savvy cultured meat laboratory startups, however, are exploring plant-based alternatives to reduce the reliance on slaughterhouse cell culture inputs, with 25 to 30 startups claiming this problem has been solved (Chriki and Hocquette 2020). Animal-derived polymers such as collagen have been used to provide a 3-D structure for myotubules (Campuzano and Pelling 2019). Synthetic or natural animal-free polymers derived from bacteria and plants have been studied as replacements, including cellulose, alginate, recombinant silk, and chitin (Campuzano and Pelling 2019), in addition to extruded hemp, maize, and pea protein (Krona et al. 2017)
Cultured Meat Nutritional Content
It is currently impossible to access all the potential issues related to nutrition in cultured meats, due to an absence of open communication in the field (Fraeye et al. 2020). Traditional meat is a rich source of high-quality protein, fatty acids, highly available iron and zinc, potassium, phosphorus, magnesium, calcium, vitamin B12, and all other B vitamins except folic acid. But so far, no strategies have been published addressing the fortification of micronutrients specific to meat—such as vitamin B12 and iron—to cultured meat (Chriki and Hocquette 2020).
It’s possible that the nutritional content of cultured meat can be controlled by adding missing nutrients to the cell culture growth media. For example, saturated fats could be replaced by other healthier fats such as omega-3s (Chriki and Hocquette 2020). Micronutrients such as vitamins and minerals in bioavailable forms may be necessary additions to cultured meat to provide nutritional parity with conventional meats.
But adding nutrients with chemical names may reduce the health halo and prevent cultured meat from achieving clean label status unless these additives are regarded as processing aids and remain off-label. And like plant-based meat alternatives, cultured meats mimicking hamburger require the addition of colorants, flavors, and texturizers to improve palatability (Fraeye et al. 2020), another no-no for a clean label.
Sustainability and Transparency
Will cultured meat really eliminate “factory farms,” or will the factory simply become a lab? Obviously, cultured meat will require less land than ruminant production, but will it require fewer carbon-emitting and energy inputs? One study found that energy requirements for cultured meat production were lower when compared with those for beef, sheep, and pork, but higher when compared with those for poultry (Tuomisto and de Mattos 2011).
Laboratories require many inputs in the form of plastic consumables. Bioreactors are not inexpensive to operate, and laboratories also have special air handling requirements. Secondary equipment such as incubators, ultra-low temperature freezers, autoclaves, and automated media handling systems all require energy inputs. And cell culture byproducts such as ammonia and lactate must be handled as a waste stream or recycled (Allan et al. 2019).
In light of increasing consumer demands for transparency, food startups may cause themselves irretrievable harm by promoting only the positives associated with new products and processes designed to alleviate concerns about meat processing and livestock production. A recent article in Modern Farmer (Vittek 2021), for example, describes cultured meat processing this way: “... the cells [are fed] a mixture of protein, vitamins and other necessary nutrients and put ... into a device called a bioreactor, which functions as a sort of womb. The cells grow, divide and eventually form into tissue, which is effectively cultured meat. It has the same nutritional value and composition as animal-based meat, but without the need to raise or slaughter animals.” Early greenwashing and unsubstantiated claims can come back to hurt a product when the media dig deeper, and more accurate reports emerge.
Cultured meat startups are currently receiving a great deal of attention and funds, including financing from such high-profile investors as Bill Gates and Richard Branson, and large multinational food companies are also backing new meat culture technologies. But do investors really want to support startups before animal welfare issues related to biopsy are addressed? Will the laboratory “yuck factor” of animal inputs, fear of technology (Goodwin and Shoulders 2013), and chemical ingredients become a marketing issue for cultured meat products? To ensure success, we must not only study new culture methods but also promote transparency in the development of cultured meat.
REFERENCES
Allan, S. J., P. A. De Bank, and M. J. Ellis. 2019. “Bioprocess Design Considerations for Cultured Meat Production With a Focus on the Expansion Bioreactor.” Front. Sustain. Food Syst. doi: 10.3389/fsufs.2019.00044.
Campuzano, S. and A. E. Pelling. 2019. “Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-Animal Sources.” Front. Sustain. Food Syst. 3: 1–9. 10.3389/fsufs.2019.00038.
Chriki, S. and J-F Hocquette. 2020. “The Myth of Cultured Meat: A Review.” Front. Nutr. 7: 1–9. doi: 10.3389/fnut.2020.00007.
FAO. 2006. Livestock’s Long Shadow–Environmental Issues and Options. Food and Agricultural Organization of the United Nations, Rome, Italy. fao.org.
Fraeye, I., M. Kratka, H. Vandenburgh, et al. 2020. “Sensorial and Nutritional Aspects of Cultured Meat in Comparison to Traditional Meat: Much to Be Inferred.” Front. Nutr. 7: 1–7. doi: 10.3389/fnut.2020.00035.
Goodwin, J. N. and C. W. Shoulders. 2013. “The Future of Meat: A Qualitative Analysis of Cultured Meat Media Coverage.” Meat Sci. 95: 445–450.
Krona, A., F. Klose, J. Gold, et al. 2017. “Developing Cultured Meat Scaffolds of Extruded Vegetable-Based Proteins.” Annual Transactions of the Nordic Rheology Society. 25: 311–313.
Melzener, L., K. E. Verzijden, A. J. Buijs, et al. 2020. “Cultured Beef: From Small Biopsy to Substantial Quantity.” J. Sci. Food Agric. doi: 10.1002/jsfa.10663.
Sharma, S., S. S. Thind, and A. Kaur. 2015. “In vitro Meat Production System: Why and How?” J. Food Sci. Technol. 52: 7599–7607.
Tuomisto, H. L. and M. J. T. de Mattos. 2011. “Environmental Impacts of Cultured Meat Production.” Environ. Sci. Technol. doi: 10.1021/es200130u.
Vittek, S. 2021. “Pet Food Companies Look Into the Future With Cell-Cultured Meat.” Modern Farmer, April 19.