Zoonutrients and Health
Understanding how molecular structures have evolved to provide nutritional functions in animals will lead to a new generation of foods that can deliver on the promise of maintaining optimal health.
Throughout history, animal products have been an important means to augment or bioconvert agricultural production to more-valuable food products. A reasonable question to ask is, “What unique nutritional value is provided by this source of food?” Are animal products simply convenient sources of protein and calories, or are there lessons that this dietary history can teach us in guiding the sciences of food and nutrition forward?
Nutrition as a science is moving beyond its previous successes in defining the essential nutrients and their absolute quantitative requirements for populations. Now the goals of scientific investigations are to build the knowledge of genetics, metabolism and biomolecules as food that will be necessary to deliver optimal health to each individual. Maintaining health sounds appealing, but it is easier to say than to achieve.
Diets that genuinely maintain health would include, for example, foods that protect individuals from exogenous stresses, toxins, and pathogens; foods that encourage optimal growth, development, maturation, and adaptation to a chosen environment; and foods that promote metabolic regulation for optimal performance.
Delivering such foods will mean not only finding food ingredients but finding new targets for the biological actions of food molecules. How will we discover these new targets? Fortunately, the evolution of animals has provided myriad examples of biomolecules that were selected for just these actions. Scientific research is finding exciting new dietary benefits that result from consuming carbohydrates that bind and eliminate toxins, proteins that influence the competitive growth of beneficial and commensal bacteria at the expense of pathogenic bacteria, and lipids that enhance the development of immune functions.
The study of these molecules and their actions reveals the remarkable structural complexity of biopolymers and biological actions that are not provided by the nonessential building blocks from which they are assembled. Understanding these structures will help to guide scientists to a new generation of foods that genuinely deliver on the promise of maintaining optimal health.
Zoonutrients
Zoonutrients are those components uniquely present in animal tissues (i.e., of the kingdom Animalia) that are consumed by other animals as food and that provide nutritional benefits beyond the provision of calories. They do not include the vitamins and minerals and other essential nutrients that cannot be synthesized by animals, but they do include:
• The essential vitamins synthesized by animals (e.g., vitamin D).
• Compounds that enhance either the stability, delivery, absorption, utilization, or actions of essential nutrients.
• Compounds that alter intestinal functions, including rate of digestion, absorption, and metabolism.
• Compounds that alter systemic metabolic responses to diet, and metabolic regulation in general.
• Compounds that alter the growth, metabolic activity, competition, pathogenicity, and toxicity of microorganisms (e.g., viruses, bacteria, molds, and fungi, and their metabolites).
• Compounds that alter the activity, absorption, secretion, and toxicity of toxins, poisons, and anti-nutritive factors.
• Compounds that are signal molecules or alter the activity of signaling systems.
• Compounds that stimulate sensory systems and alter the experience and consequences of eating.
There are many examples of animal-based food products that take advantage of the selective accumulation of essential nutrients by animals, and that thus represent an unusual and selective source of phytonutrients, but these are not considered to be zoonutrients. However, some animal tissues both concentrate and modify the structures of essential nutrients, and these modified nutrients are examples of zoonutrients.
Zoonutrients discussed here are those present in foods routinely consumed, including products from invertebrates, fish, reptiles, avians, and mammals. This article is meant not as an endorsement for or against any particular eating choice, but as a description of the scientific advances that are emerging as investigators seek to understand the biological value of under-characterized food materials.
--- PAGE BREAK ---
Basis for Nutritional Action
Three types of zoonutrients are considered: those nutrients that are expressly designed to be consumed by offspring of the animal; those nutrients that are present in tissues of animals and provide their normal actions or functions when consumed as food; and those nutrients that are present in animal tissues with particular functions but that provide distinctly different actions when consumed as food.
The fact that various animal tissues evolved to produce food for offspring has been acknowledged by nutritional researchers for decades (Blackburn, 1992). Even insects produce compounds that provide nutrition for their young, and secrete compounds that can profoundly alter the growth, development, and maturation of offspring. Examples are honey and royal jelly produced by honeybees. Fish and reptiles utilize a variety of strategies to provide nourishment to their offspring, with the obvious food example being the mixture of nutrients within the egg.
In addition to the biologically active components of avian eggs, avian crop fluids contain interesting catalytic activities and proteins, although, unlike eggs, these components and their nutritional properties have not been exploited commercially. In mammals, specific processes that produce zoonutrients abound. The most obvious example is the secretion of milk for the sole purpose of nourishing an infant (Table 1).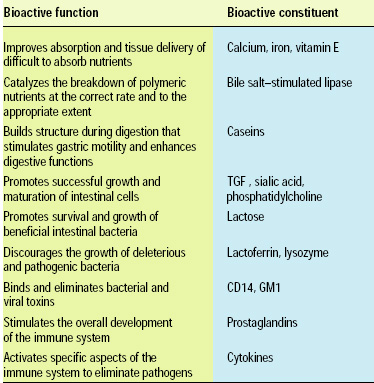
In the above illustrations, the biomaterials produced by animals are not simply carriers of absorbed and/or partly digested plant material—they include novel molecules that derive from the animal’s genetic and metabolic machinery. Darwinian selective pressure was in many cases acting intensively on the elaboration of nourishing compounds within these materials. This evolutionary process has led to the production of literally dozens of discrete molecules that act to enhance the probability that healthy offspring survive to reproduce.
Milk provides vivid examples of the consequences of evolution to elaborate biological polymers with nourishing properties beyond essential nutrients. With the arrival of modern genetic tools, and in particular comparative and functional genomics, lessons are being learned from a variety of animals and span the range of biopolymer classes, proteins, carbohydrates, and lipids.
Zoonutrient Actions and Benefits
In the traditional views of health, nutritional diseases are caused by explicit deficiencies of essential nutrients. The simple provision of those deficient nutrients generally cures the disease. Similarly, deficiency diseases are prevented in a population by ensuring adequate routine intakes of foods containing all the essential nutrients. Nutrition and food science have been successful in addressing the biological challenges of understanding the essential nutrients and their deficiency, from characterizing the biochemical functions of the essential nutrients to establishing a recommended daily allowance for each, now revised as dietary reference intakes.
Now scientists are refocusing their research to address diet and health beyond the essential nutrients and their deficiency. The goals of modern diet and health research are increasingly (1) to promote optimal metabolic regulation and prevent diseases that emerge from chronic imbalances in metabolism, such as obesity, heart disease, osteoporosis, and type 2 diabetes; (2) encourage growth and development (imprinting) within each individual’s environment, i.e., promote successful immune responses to endemic pathogens but also to avoid intolerances and allergies to routine and benign components of the immediate environment; and (3) protect individuals from pathogens, toxins, and stresses. To achieve success in this next generation of diet-related health issues requires a distinctly different strategy from that used to success in discovering essential nutrients.
In many respects, discovering essential nutrients was easy: eliminate a suspected nutrient from the diet of animals, and if it is essential, deficiency will result in measurable impairment in phenotype in all individual in all circumstances. In contrast, nonessential health benefits will not necessarily provide measurable benefits in all individuals nor in all situations. In the most simple example, a nutrient that is protective from a particular stress is only detectable, or even valuable, when the individual is exposed to that stress. Clearly, nutrition research must adapt new strategies to identify nutrients that are beneficial and to understand why and when they provide their benefit.
Fortunately, as a result of the selective pressure throughout evolution to produce such molecules in animals, zoonutrients are providing novel targets for scientific research and new molecular strategies to develop innovative foods. The lessons learned will be useful in discovering and delivering new food ingredients from all possible food sources, plants, microbes, and animals.
--- PAGE BREAK ---
• Metabolic Regulation. Diseases that result from long-term chronic imbalances in metabolism are not due to deficiencies of essential nutrients, do not necessarily produce biomarkers of damage until the disease is well established, and are not resolved by the simple provision of a limiting nutrient. Preventing diseases that are the result of chronic metabolic dysregulation means maintaining normal metabolism, thus avoiding problems before the damage occurs, i.e., before any disease has developed.
The principle that nonessential nutrients contribute to the regulation of an individual’s metabolism has been well established by the last century’s successes in describing the tendency of particular saturated fatty acids in the diet to raise LDL cholesterol in certain individuals. It is perhaps surprising that the successes gained by this understanding have not led more rapidly to a broader and more quantitative perspective of metabolic health (Watkins et al., 2001). Nevertheless, personal assessment of metabolism is becoming recognized to be the next breakthrough in public health management (Watkins et al., 2002).
• Metabolic Imprinting. Animals and especially humans possess a broad physiologic and metabolic plasticity. That is, they have an astonishing capacity to “learn” features about their environment and to build structures and optimize biochemical pathways to accommodate specifically to the unique properties of each individual’s environment. Obvious examples that scientists are actively researching are the complex systems of olfactory preference in which each of us “learns” what odors characterize foods that are good and bad for us, and the processes of specific immunity in which an individual’s immune system must “learn” what molecules are self and safe and what are foreign and unsafe. By their design, when these maturation or imprinting processes are successful, we find delight in healthy foods and distaste in unhealthy foods. We mount an immediate and appropriate immune response to foodborne pathogens but develop tolerance to non-pathogenic bacteria and food materials. If maturation goes wrong, we find less (or no) delight in healthy foods and develop intolerances and allergies to otherwise healthy ingredients.
Ideally, our foods should enhance the processes of learning and adaptation, sensitize us appropriately, and encourage the optimal adaptive maturation to novel environments. First, we must understand how food can affect these processes. Certain of the zoonutrients provide a potential information gold mine to teach scientists about the molecules and how they work.
• Hazard Protection. Many of the health issues of modern humans relate to the fact that we are routinely exposed to a variety of stresses, bacterial pathogens, toxins, and pollutants. These stresses, even when not causing immediate disease, affect chronic damage and can eventually lead to intestinal impairment and dysfunction and to inappropriate responses such as inflammation. A valuable benefit of foods would be to provide agents, compounds, or structures that could interrupt such stresses, even prevent them from contacting the intestinal targets of their damage and toxicity.
The basic molecular strategies for such protection can be seen in the salivary excretions of animals, intestinal products themselves, and the epithelial secretions of the lactating mammary gland. In each case, remarkably clever mechanisms have evolved to interrupt, bind, entangle, or catalytically degrade toxins and prevent them from exerting a negative influence on an animal’s health.
Perhaps the most ingenious biological strategy yet discovered is the way in which the growth of beneficial microorganisms is encouraged by providing them selective nutrients as an additional means to enlist a symbiotic organism to assist the host in protection. Research into the benefits of milk is even demonstrating that beneficial bacteria that are encouraged by milk components assist to coordinate the most appropriate host response to stress and foreign and deleterious organisms (reviewed in German et al., 2002).
Nutrition research is moving forward with the objectives of maintaining optimal metabolism, stimulating appropriate development, and providing active protection. The fields of food and nutrition are thus broadening their influence dramatically beyond issues of essential nutrients and are supplying the means through scientific knowledge to maintain health and prevent disease. In this new health paradigm, the molecular properties of foods and food components are also being reexamined. However, preventing metabolic and pathogenic disease is not the same thing as ensuring nutrient adequacy. Both research and its application will invariably be more personal and more immediate. An essential nutrient is essential for everyone. A specific disregulation of metabolism may only occur in some individuals and only at certain times. Profiting from the research discoveries of modern nutrition will require a new and more intimate, i.e., personal, perspective on the interaction of diet and health. The rewards promise to be substantial.
--- PAGE BREAK ---
Structures and Functions
The basic building blocks of food—amino acids, fatty acids, sugars, and nucleotides—cannot be considered as derived exclusively from animals and are therefore not zoonutrients. However, the macromolecules and polymers formed from these basic building blocks are the direct result of animal genes and metabolism and thus represent the molecular legacy of the evolution of structure and function within animals.
Research to establish that these structures provide nutritional or biological value beyond the subunits that they contain has been painstaking and complex. Complete investigations conducted on isolated molecules or cells to establish mechanisms, on whole animals to validate these mechanisms in vivo, and on humans to confirm their final benefits are still rare. Nevertheless, sufficient demonstrations exist to provide confidence that the structures and actions discovered represent proofs of principle and argue for more intensive research.
• Amino acids. Animal products have long been recognized to be an excellent source of protein because of the close match between the amino acid composition of the products and the amino acid needs of animals. In addition to the basic amino acids needed for protein synthesis, animals contain novel amino acids, such as taurine, that are found rarely or not at all in the plant kingdom. Despite evidence that taurine affects a variety of metabolic processes, from cholesterol levels to immune function to digestion, there is no current detailed understanding of the mechanism by which these effects occur (Lombardini and Schaffer, 2002). Taurine is a methylene substituted sulfonic acid in millimolar quantities in many tissues of the body but not incorporated into proteins. It is an essential amino acid for cats, the only animal that cannot synthesize it, yet benefits of taurine supplementation have been documented in a variety of human diseases. Thus, as a zoonutrient, taurine seems to provide benefit by supplementing the amounts produced by endogenous biosynthesis.
Creatine, an amino acid derivative, is ubiquitous in animal tissues, yet the structure/function mechanisms for this molecule remain poorly understood. Because it is a supplementary source of activated phosphate, creatine monophosphate has been proposed to extend the capacity of a cell’s ATP pool and to prolong short-burst muscle activities (Volek et al., 1997). Since humans have the ability to synthesize creatine, it is not clear how an exogenous supply of this nutrient should increase tissue concentrations. Nevertheless, under certain conditions, creatine supplementation appears to produce metabolic effects consistent with improved performance.
Another amino acid derivative that provides health benefits in certain populations is the zwitterionic quarternary amine, carnitine. Carnitine can be synthesized de novo from lysine or methionine, or obtained from dietary sources including meat, fish, poultry, and milk. It is known to function to transport organic acids across membranes, and dysfunctional fatty acid metabolism is associated with defects in this transport system. Again, increased dietary carnitine appears to influence such processes (Krajcovicova-Kudlackova et al., 2000). Recently, dietary carnitine was reported to act synergistically with α-lipoic acid in restoring mitochondrial function in old mice (Liu et al., 2002).
• Peptides. Over the past decade, numerous studies have shown that an astonishing number of peptides, when consumed in foods, produce metabolic effects that are not shown by their constituent amino acids alone. These peptides include those present intact in the natural tissues or biomaterials from animals and those peptides that are part of existing animal proteins but are released by proteolysis during digestion. Bioactive peptides have been characterized in milk, eggs, muscle, and other tissues.
The actions of these molecules as studied in vitro are quite diverse, some binding metals, cofactors, or toxins; some producing antibiotic effects toward microbial pathogens; and some even acting on signal transduction pathways on target cells in the intestine and peripheral tissues. Different peptides from milk have been well described to modify blood pressure, neurologic activity, immune functions, food intake, intestinal functions, and even dental calcification (Shah, 2000).
These discoveries have prompted scientists to pursue the mechanistic targets of action to develop greater understanding of how specific molecules in foods affect health. Special attention is now being paid to peptides produced by proteolysis, such as the lactoferricin fragment from lactoferrin, revealing bactericidal activity, probiotic-stimulating activity, and anti-viral and anti-inflammatory actions. Again, discoveries of these actions from human milk have led scientists to explore how foods modulate the immune system and the interaction between beneficial bacteria, pathogenic bacteria, and our innate and acquired immune-protective mechanisms (Donnet-Hughes et al., 2002).
--- PAGE BREAK ---
• Proteins. Zoonutrient proteins, such as the milk caseins and egg albumins, are accepted storage/delivery forms of amino acids for the growing animal, but how their composition and structure are enhanced in animal proteins for nutritional value is still being resolved. The caseins precipitate and form a gastric gel in response to the cleavage of one peptide bond by a highly selective gastric enzyme—a process clearly the result of evolutionary protein engineering. The ways that structures resulting from this engineering facilitate digestive function and the net delivery of amino acids to blood and growing tissues are still being revealed by nutritionists (Dangin et al., 2002).
However, such examples are not solely discovered in milk. Another example of this type of secondary organization occurs in the major proteins of the larval jelly of honeybees. Two of the five homologous proteins in this class of proteins contain tandem repeats of nitrogen-rich amino acids, thus providing the larvae with high concentrations of bioavailable nitrogen (Schmitzova et al., 1997).
A comprehensive summary of all studies on the biological properties of milk proteins is beyond the scope of this article. However, understanding how biological processes can be modified by the consumption of specific proteins is stimulating researchers to broaden their perspectives on nutritional actions.
Initially, food and nutrition researchers examined the biological properties of food ingredients as pharmaceuticals, testing ingredients against targets of existing disease. Some candidates emerged, notably antimicrobial and anti-hypertensive peptides. Numerous proteins in milk and other animal fluids exhibit antimicrobial activity with complementary mechanistic actions. One of the better-characterized milk proteins is lysozyme, an enzyme that catalyzes hydrolysis of the linkage between N-acetylglucosamine and N-acetylmuramic acid in bacterial cell walls. This enzyme, along with other milk proteins involved in host defense, such as transferrin, peroxidase, and immunoglobins, is also produced in a variety of other exocrine secretions such as tears and sweat (Blackburn, 1992). Certainly the poster-child of nutritious proteins is lactoferrin present in milk. This single protein has been reported to provide protection from viral infection, bacterial infection, fungal infection, and lipid oxidation; to reduce inflammation; to promote beneficial bacterial growth; and to enhance iron absorption and immune development and response (Weinberg, 2002; van der Strate, 2001).
Although the evolution of the products of the mammary gland cannot be deduced simply from phylogenetic comparisons, clues are emerging. The major whey protein, α-lactalbumin, is paralogous (divergence of different genes during evolution afer duplication of the same original gene) to lysozyme, and probably arose from a gene duplication before the divergence of birds from mammals. The molecular function of α-lactalbumin in the synthesis of lactose is to change the specificity of UDP galactosyltransferase. Thus, instead of adding galactose to a glycoprotein, it is added to glucose, forming lactose. Since there is an excess of α-lactalbumin beyond that needed for milk lactose synthesis, and since the amino acid profile of the enzyme is similar to that estimated to be required for an infant, this protein serves a nutritive function as well.
Genomic tools can now be leveraged to explore the biological information embedded in the genetic sequences that are responsible for the synthesis, assembly, and secretion of mammalian milks and other zoonutrient sources.
Not all nutritionally valuable components in animal tissues are due to evolutionary selection for their food value and are presumably nutritionally active through serendipity. A well-documented example of the nutritional benefit of consuming a muscle-derived zoonutrient protein is in heme and iron absorption. Whereas heme absorption is relatively unaffected by other dietary constituents, non-heme iron absorption is highly sensitive to the food matrix (Conrad and Umbreit, 2000). Perhaps not suprisingly, other factors in meat, as yet uncharacterized, also contribute to the bioavailability of iron even in the absence of heme, and the iron status of societies who are able to acquire animal products in their diet is in general superior to that of those who cannot.
• Oligosaccharides. There has been a dramatic increase in research activity in the field of carbohydrate biology (glycobiology) over the past five years as the pivotal roles of saccharides have emerged in cell signaling, cell recognition, metabolic regulation, and cell protection. Scientists are beginning to realize that sugars, oligosaccharides, and polysaccharides exhibit high structural specificity, as do proteins and polynucleotides, and with the ability to appreciate the complexity of structure, scientists have begun to appreciate the complexity of function. The question nutritionists are now asking is, “Does structure matter to the actions of saccharides in foods?”
Historically, complex carbohydrates were recognized for their nutritional value, but this value was initially thought to be associated with plant cell wall material and the nonspecific benefits of indigestible fibers to the intestine. The discovery of oligosaccharides in milk prompted a reevaluation of the health benefits of consuming complex carbohydrates (Kunz et al., 2000; Urishima et al., 2001). Discovery of these oligosaccharides has illuminated intestinal functions in humans and possible benefits of foods that were not previously considered. A brief sampling of the types of oligosaccharides in animals illustrates the breadth of the potential benefits (Table 2).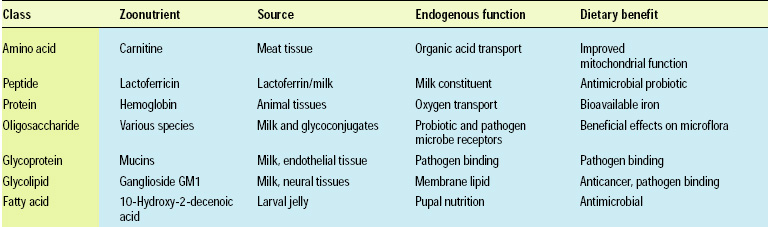
--- PAGE BREAK ---
Dozens of highly specific oligosaccharides are present at relatively high concentrations in human milk. More than 80 acidic and neutral oligosaccharide species have been identified and characterized, and many are synthesized by the same glycosyltransferases as the glycoprotein and glycolipid cell surface receptors from somatic cells.
Molecules that are an intricate part of an animal’s biology, such as the lysozyme discussed above, are incorporated into the milk delivered to the neonate. Studies showing that oligosaccharides in human milk are not metabolized or absorbed in the small intestine confirm that they are not a source of calories for the infant, but instead for the microflora. The composition and structure of these molecules and their properties as selective prebiotics, complexing agents, and polymers that bind and neutralize intestinal pathogens have been shown (Kunz et al., 2000). Recent research even demonstrates that immunologically active oligosaccharides from human milk are excreted in infant urine confirming that they reach and presumably act in blood and peripheral tissues (Kunz et al 2000). In each case the highly specific structures imparted by the animal enzymes that synthesize them are critical to their actions as food ingredients.
• Polysaccharides. Animal cells produce relatively few polysaccharides because they do not construct cell walls. Nevertheless, polysaccharides are produced by animals, particularly by specialized epithelial cells, for highly specific extracellular matrices. The most abundant of these structural polysaccharides are the mucins, extracellular polysaccharide–protein conjugates in which a complex polysaccharide network is anchored to a membrane-bound protein core. The diverse chemical structures of the different mucins are still not fully described, nor their functions as the surfaces of epithelial cells, much less their actions as food ingredients. However, scientists are now exploring the actions of the mucins present on milk fat globules as a model, and multiple actions are suggested (Patton, 2001). These actions can be broadly described as prebiotic stimulation of the growth of beneficial bacteria, complexation and elimination of deleterious and pathogenic bacteria, and scavenging of toxins.
The establishment of a stable, protective, and non-inflammatory microflora is one of milk’s most interesting but poorly understood benefits. Only recently has the role of milk’s mucins been suggested to be one of the active ingredients achieving this effect. Beneficial lactic acid bacteria that colonize the lower intestine of infants remain attached to intestinal mucins. Not only do these bacteria attach to the intestinal mucins, but the competitive advantage provided for specifically beneficial bacteria is in part due to their synthesis of mucinases that allow them to use these molecules as substrate and thus in many respects approach a symbiotic relationship with the host.
From this viewpoint, milk, by providing mucins that selectively enrich the infant microflora environment with fermentation substrate encouraging the growth of beneficial bacteria is a logical though very innovative nutritional strategy. At the same time though such a nutritional benefit is experimentally difficult to confirm. Whether provision of milk mucins would be beneficial throughout life is not known; however, following episodes of microfloral disruption (antibiotic treatment, intestinal infections, etc.), these nutrients may play roles similar to those played in infants.
• Fatty Acids. Animals generally do not synthesize a wide range of fatty acids and in particular are unable to make the essential polyunsaturated fatty acids (PUFAs), which are obtained ultimately from plant materials. However, animals exhibit a significant ability to both metabolize and accumulate long-chain PUFAs as storage lipids. As a result, many animals accumulate nutritionally important concentrations of particular fatty acids.
The accumulation of long-chain n-3 PUFAs in fish is the best-studied example of a nutritional benefit derived from the accumulation of fatty acids in animal tissues. When it was observed epidemiologically that consuming fish was associated with altered disease incidence, scientific research raced ahead showing how consuming n-3 PUFAs was beneficial. Now that the biological effects of consuming these fatty acids from wild fish are recognized, they are being produced industrially in plants and animals for food.
Although higher animals do not typically synthesize unusual fatty acids, lower animals do. An interesting and unique fatty acid is present in the larval jelly of honeybees. Whereas most fatty acids in plant and animal tissues are 14–20 carbons long, the unesterified royal jelly fatty acids are 8–10 carbons long and often contain hydroxyls or a second carboxyl moiety. The most prevalent is 10-hydroxy-2-decenoic acid, and royal jelly is the only documented natural source of this fatty acid.
--- PAGE BREAK ---
As the fatty acids of royal jelly are short and unesterified, one might expect them to be involved in signaling pathways at the expense of any structural role. For example, antimicrobial properties were ascribed to 10-hydroxy-2-decenoic acid, and the beneficial effect is believed to result from its role in cell signaling pathways (Pollet et al., 2002).
An interesting example of the importance of the positional specificity of fatty acids can be found in the triglycerides of milk. Plants esterify unsaturated fatty acids in the sn-2 position of triglycerides, yet milk triglycerides contain high levels of the most abundant saturated fatty acid palmitic acid, in the sn-2 position. A body of literature now demonstrates that the absorption of palmitic acid and calcium is increased by this specific structure (Kennedy et al., 1999).
• Phospholipids and Glycolipids. Complex lipids are the basic building blocks of biological membranes. Most phospholipids are similar between plants and animals although animals tend to accumulate long chain PUFA in the sn-2 position of phospholipids, providing a highly concentrated dietary source of these fatty acids. Glycolipids, however are in some cases very specific in the structure of the carbohydrate moiety of the molecule. The highly specific structures of glycolipids have been studied for several years because of the discovery of the ability of gangliosides to bind to specific pathogenic microorganisms (Table 2, 3). The structural specificity of these glycolipids is quite profound, however. Hence the gangliosides of human milk are not found or are found in lower abundance in bovine and other milks that are consumed routinely by humans. Therefore, translating the benefits of gangliosides from humans into foods for humans will not be as simple as consuming bovine milk products. Strategies that take the knowledge of the stuctural specificity of the binding to produce these molecules industrially is now being pursued for both food ingredients and pharmaceuticals.
Future Research Directions
Scientists are assembling the human genome and genomes of animals, plants and microorganisms into functionally annotated databases. Following close behind are increasingly annotated databases of proteins (proteomes) and metabolites (metabolomes) as biological science truly leaps into the information age.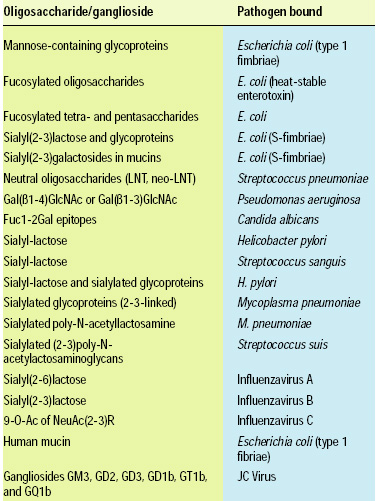
Food science and nutrition will use information in several ways. Genome comparison between humans and animals can follow the evolutionary emergence of zoonutrients, their structures and functions and targets. Database searches use these molecules and targets to detect similar molecules or catalytic activities in plants and microorganisms.
The technologies of genomics are also making it possible to investigate nutritional questions in new ways, to investigate more complex effects of non-essential nutrients whose mechanisms and even benefits are not yet known or which could be acting at multiple targets.
DNA arrays, and metabolite profiling seek to measure the expression of all the genes and metabolites of an organism (fluid or tissue or cell) simultaneously. It is possible to confirm specific, hypothesized responses and, simultaneously, explore all other genes and metabolites to determine if the nutrient produced unanticipated effects, both positive and negative.
This article has emphasized the properties of zoonutrient components of the diet for their health-promoting properties. However, animal products are luxurious foods highly appreciated for many desirable food functionalities, including flavor, taste, structure, texture, mouthfeel, and overall pleasure. Structural knowledge of the molecules of animal tissues and fluids will eventually be mapped onto these other functionalities, making it possible to understand how each function can be delivered more effectively as food.
The challenges to foods are great, but so are the opportunities. As scientists look throughout the biological information expanding all around them, it becomes important to choose which path to follow. Which organisms can show us? Animals evolved many health-promoting food ingredients for their offspring, providing interesting scientific paths toward health.
Each step we take will move toward the goal of building the knowledge of foods and consumers with sufficient molecular clarity to provide all food attributes: delightful, convenient, and affordable foods that enhance the health of each individual consumer.
by Robert E. Ward and J. Bruce German
The authors are, respectively, Research Assistant and Professor, Dept. of Food Science and Technology, University of California, 1 Shields Ave., Davis, CA 95616. Author German is on sabbatical at Nestlé Research Center, P.O. Box 44, CH-1000 Lausanne, Switzerland. Send reprint requests to author German.
The authors gratefully acknowledge the support of the California Dairy Foundation for author Ward and the insightful comments and suggestions by Cora Morgan.
References
Blackburn, D.G. 1992. Lactation: Historical patterns and potential for manipulation. J. Dairy Sci. 76: 3195-212.
Conrad, M.E. and Umbreit, J.N. 2000. Iron absorption and transport-an update. Am. J. Hematol. 64: 287-298.
Dangin, M., Boirie, Y., Guillet, C., and Beaufrere, B. 2002. Influence of the protein digestion rate on protein turnover in young and elderly subjects J. Nutr. 132: 3228S-3233S.
Donnet-Hughes, A., Duc, N., Serrant, P., Vidal, K., and Schiffrin, E.J. 2000. Bioactive molecules in milk and their role in health and disease: The role of transforming growth factor-beta. Immunol. Cell. Biol. 78(1): 74-79.
German, J.B., Dillard, C.J., and Ward, R.F. 2002. Bioactives in milk. Current Opinion Clin. Nutr. Metabolic Care 5: 653-658.
Gobbo, M., Biondi, L., Filira, F., Gennaro, R., Benincasa, M., Scolaro, B., and Rocchi, R. 2002. Antimicrobial peptides: Synthesis and antibacterial activity of linear and cyclic drosocin and apidaecin 1b analogues. J. Med. Chem. 45: 4494-4504.
Kennedy, K., Fewtrell, M.S., Morley, R., Abbott, R., Quinlan, P.T., Wells, J.C., Bindels, J.G., and Lucas, A. 1999. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: Effects on stool biochemistry, stool characteristics, and bone mineralization. Am. J. Clin. Nutr. 70: 920-927.
Komagome, R., Sawa, H., Suzuki, T., Suzuki, Y., Tanaka, S., Atwood, W.J., and Nagashima, K. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 76: 12992-13000.
Krajcovicova-Kudlackova, M., Simoncic, R., Bederova, A., Babinska, K., and Beder, I. 2000. Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 49: 399-402.
Kunz, C., Rudloff, S., Baier, W., Klein, N., and Strobel, S. 2000. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Ann. Rev. Nutr. 20: 699-722.
Liu, J., Head, E., Gharib, A.M., Yuan, W., Ingersoll, R.T., Hagen, T.M., Cotman, C.W., and Ames, B.N. 2002. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-L-carnitine and/or R-alphalipoic acid. Proc. Natl. Acad. Sci. 99: 2356-2361.
Lombardini, J.B. and Schaffer, S.W. 2002. Special Issue: Taurine: Discovered 185 years ago and still intrigues the scientific community. Amino Acids 23: 343.
Martin-Sosa, S., Martin, M.-J., and Hueso, P. 2002. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 132: 3067-3072.
Patton S. 2001. MUC1 and MUC-X, epithelial mucins of breast and milk. Adv. Exp. Med. Biol. 501: 35-45.
Pollet, S., Bottex-Gauthier, C., Li, M., Potier, P., Favier, A., and Vidal, D. 2002. Insight into some of the signaling pathways triggered by a lipid immunomodulator. Immunopharmacol. Immunotoxicol. 24: 527-546.
Schmitzova, J., Klaudiny, J., Albert, S., Schroder, W., Schreckengost, W., Hanes, J., Judova, J., and Simuth, J. 1998. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. 54:1020-1030.
Shah, N.P. 2000.Effects of milk-derived bioactives: An overview. Brit. J. Nutr. 84(Suppl 1): S3-S10.
Urashima, T., Saito, T., Nakamura, T., and Messer, M. 2001. Oligosaccharides of milk and colostrum in non-human mammals Glycoconj J. 18: 357-371.
van der Strate, B.W., Beljaars, L., Molema, G., Harmsen, M.C., and Meijer, D.K. 2001. Antiviral activities of lactoferrin. Antiviral Res. 52: 225-239.
Volek, J.S., Kraemer, W.J., Bush, J.A., Boetes, M., Incledon, T., Clark, K.L., and Lynch, J.M. 1997. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J. Am. Dietet. Assn. 97: 765-770.
Watkins, S.M., Hammock, B.D., Newman, J.W., and German J.B. 2001. Individual metabolism should guide agriculture toward foods for improved health and nutrition. Am. J. Clin. Nutr. 74: 283-286.
Watkins, S.M. and German, J.B. 2002. Metabolomics and biochemical profiling in drug discovery and development. Current Opinion Molec. Ther. 4: 224-228.
Weinberg, E.D. 2001.Human lactoferrin: A novel therapeutic with broad spectrum potential. J. Pharm. Pharmacol. 53: 1303-1310.
