
Tweaking Nature's Menu: Plant Genome Editing
New gene-editing tools usher in an exciting era of plant breeding.
Article Content
From the humble experiments in the garden of an Austrian monk to the mapping of eukaryotic genomes, humans have been tinkering with genetic instructions for centuries, trying to unravel the mysteries of life and edit the course it follows. Agriculture has facilitated the field of genetics along with its innovation and application. In reciprocation, the field of genetics has empowered agriculture to provide a highly diverse food supply that meets the needs of a changing world, introducing new ways to increase crop yields, improve the nutrition value of crops, enhance their tolerance to insects and disease, and feed the world.
Most of the foundational information about genes and hereditary traits would probably not exist without the work of botanist Gregor Mendel. In the mid-19th century, Mendel, an Austrian monk, discovered the principles of heredity by tinkering with pea plants in his garden. Prior to Mendel’s experiments, selective plant breeding had been occurring casually for thousands of years but was based simply on selecting varieties of plants that exhibited desirable traits, such as climate suitability and high yield, and using their seeds to grow future generations. Through cross-fertilizing many varieties of pea plants with contrasting traits, Mendel made important discoveries about genes and heredity: the law of segregation, which specifies that each inherited trait is determined by a gene pair containing one allele from each parent; the law of independent assortment, which indicates that genes for different traits assort independently from each other; and the law of dominance, which states that in organisms with alternate forms of a gene, the dominant trait is expressed. At the time of his discoveries, Mendel did not know that these genetic rules were applicable not only to plants and certain traits but to nearly all living species and hereditary traits. In essence, all living things—including plants— have parents, and the offspring of parents inherit their genes. Through artificial selection and cross-fertilization, Mendel paved the way for plant breeding that has evolved over many decades to systems that are more precise and efficient.
The Classics
The most common and longest era of plant breeding is traditional or conventional plant breeding, which began centuries ago by simply selecting and cultivating the seeds of plants with desirable traits. This process requires years of planting different seed varieties, growing plants to full term, examining them to see which plants survive and possess the most desirable characteristics, and saving the seeds to plant in subsequent seasons. Upon reevaluating (or rediscovering) the work of Mendel, botanists and plant breeders learned how to selectively crossbreed plants, reducing the time necessary to yield plants with desired characteristics. Crossbreeding involves a series of steps that take over the natural reproduction activities of plants, which can be asexual or sexual. Asexual reproduction occurs when a part of one parent plant detaches and grows to become a complete plant; this process yields plants that are genetically identical to the parent plant. Sexual reproduction occurs when male gametes (pollen) of plants fuse with female gametes (ovules) of plants. During selective crossbreeding, or artificial selection, plant breeders collect pollen from the flower of one plant with desired traits and transfer it to the flower of another plant of the same or similar species that has other desirable traits. Some of the offspring of cross-pollinated plants will exhibit beneficial traits; others will not. The breeders then select which progeny produced by artificial selection to continue growing.
Simple selection and artificial selection are both traditional plant breeding techniques that have allowed humans to exert some control over the traits of different plant species for hundreds of years. That level of control, however, is not absolute. While humans can choose which plants to grow and which plants to crossbreed, the precise makeup of the genes of the plant is still unpredictable. As the law of assortment dictates, the genes of parents assort randomly in offspring. In addition, a permanent alteration in the deoxyribonucleic acid (DNA) sequence of a gene, called a mutation, can randomly occur. Thus, traditional plant breeding techniques yield plants that possess desirable traits as well as plants that possess undesirable traits. Most of the crops growing today are the result of conventional breeding techniques utilizing selection or artificial selection. Examples include corn, wheat, apples, bananas, strawberries, tomatoes, cucumbers, broccoli, brussels sprouts, and squash.
 A World of Mutants
A World of Mutants
Although artificial selection reduced the time involved in conventional plant breeding, the process can nonetheless take up to 25 years to produce a new crop variety with specific characteristics, and the level of control over gene inheritance and trait expression is still somewhat unpredictable. The unpredictability of traditional plant breeding is a natural consequence of assortative gene pairing and/or spontaneous changes—mutations—in the DNA sequence of plants. As essential tools of evolution, mutations alter the appearance and other properties of plants and other organisms, resulting in new varieties and thereby expanding biodiversity. In nature, they occur randomly in response to external factors, such as disease or pests, or internal factors, such as metabolites; such mutations enable plants to adapt to their environment. Many plant mutations are of no use to plant breeders, but in rare instances, spontaneous plant mutations have yielded varieties of plants with attractive novel characteristics (e.g., red Bartlett pears). Because of this, plant scientists have been inducing mutations in plants with radiation or mutagenic chemicals since the 1930s. In addition, “classical breeding does not have an easy way to remove undesired traits. Often chemicals or radiation would be used to induce random mutations to attempt to remove an undesired trait,” says Scott Jackson, a professor with the Institute of Plant Breeding, Genetics, and Genomics at the University of Georgia. Today, more than 3,000 crops are mutant plants, including red Bartlett pears; ruby red grapefruit; and varieties of bananas, barley, cotton, lettuce, peanuts, peas, sorghum, and wheat (FAO/IAEA 2017). In fact, bread and pasta are made with mutant wheat, and beer and whiskey are made with mutant barley. When combined with selection and detection, plant mutations—both spontaneous and induced—are the most inexpensive way to diversify crop varieties.
 Simple selection, crossbreeding, artificial selection, and mutation breeding—all of which are considered conventional or classic plant breeding—have been the dominant ways to alter or edit the genetic material of plants. These methods helped facilitate the Green Revolution between the 1940s and 1960s, during which the productivity of global agriculture increased exponentially. Plant scientist Norman Borlaug, the father of the Green Revolution, and other scientists used selection, crossbreeding, and mutation breeding to develop grain varieties that were high-yielding, disease-resistant, and adaptable to different climate conditions and agrochemicals, helping to reduce famine and starvation around the world. That’s an exceptional feat, but as the world’s population continues to grow and pests (diseases and insects) become more adaptable and aggressive against existing crops and technologies, continual improvements in crop breeding need to occur at a quicker pace. By 2050, the global population is projected to be 9.6 billion people; therefore, the amount of food grown in the next 40 years must equal the total amount of food grown in the past 10,000 years.
Simple selection, crossbreeding, artificial selection, and mutation breeding—all of which are considered conventional or classic plant breeding—have been the dominant ways to alter or edit the genetic material of plants. These methods helped facilitate the Green Revolution between the 1940s and 1960s, during which the productivity of global agriculture increased exponentially. Plant scientist Norman Borlaug, the father of the Green Revolution, and other scientists used selection, crossbreeding, and mutation breeding to develop grain varieties that were high-yielding, disease-resistant, and adaptable to different climate conditions and agrochemicals, helping to reduce famine and starvation around the world. That’s an exceptional feat, but as the world’s population continues to grow and pests (diseases and insects) become more adaptable and aggressive against existing crops and technologies, continual improvements in crop breeding need to occur at a quicker pace. By 2050, the global population is projected to be 9.6 billion people; therefore, the amount of food grown in the next 40 years must equal the total amount of food grown in the past 10,000 years.
Phenotype Versus Genotype
Conventional plant breeding has always relied heavily on evaluation of phenotypes (Pérez-de-Castro et al. 2012). The phenotype is the physical (i.e., observable) expression of genetic traits (e.g., colors of flowers, size and shape of fruits, height of stalk, etc.), and waiting for different varieties of plants to grow to determine whether plants exhibit desirable physical traits is a lengthy process. However, since the discovery in the 1950s of the structure of DNA and how DNA operates, a series of technological advancements in genomics are poised to usher in another revolution in plant breeding that could make plant breeding more precise, more effective, and higher yielding. These advancements are based on evaluating the genotypes (DNA sequence) of plants and purposefully manipulating or modifying their genetic codes. “Genome-editing technology has rapidly moved to the forefront as a key approach for identification of gene function and for development of useful traits,” says Jeffrey Wolt, a professor and co-director of the Crop Bioengineering Center at Iowa State University. In addition, they improve the likelihood of growing a specific crop in a variety of environmental conditions. “Breeding for genotype allows for more rapid, efficient, and selective identification and movement of traits into preferred germplasm for plant improvement,” Wolt explains. “[It] augments rather than supplants breeding for phenotype since selections made through genotyping must be finished through conventional breeding and, very importantly, must evaluate the performance of the derived phenotype in terms of the breeding objectives [that] are being sought.”
The first plant to be modified through deliberate genetic modification, or genetic engineering, was an antibiotic-resistant tobacco plant. It was created in 1982 by transferring certain genes from one species (a bacterium) into the genome of another species (a tobacco plant); this process is called recombinant DNA technology or transgenic gene editing. It allows scientists to introduce the genes of nearly any organism into the genome of a completely unrelated organism—something that almost never occurs in nature. Recombinant DNA technology prevented the total collapse of Hawaii’s papaya industry from papaya ringspot virus. By transferring a single gene from the virus into a papaya’s genome, researchers at Cornell University developed a papaya variety that was resistant to the pathogen. Transgenic gene editing “can be faster [than traditional breeding] because it typically would not require backcrosses and you can make multiple changes at once,” says Alex Schultink, a postdoctoral scholar at the Innovative Genomics Institute at the University of California, Berkeley. “It’s safer because you have a better understanding of what genes you’re changing and what affects they might have. It’s more powerful because you have access to alleles not present in the breeding collection.” Wolt provides another rationale for transgenic gene editing: “Classical plant breeding requires significant backcrossing to move a desired trait into a preferred germplasm for varietal development. This process is costly [and] time-consuming and inevitably involves the introduction of some less desirable traits into the preferred germplasm along with the desired trait,” he says. “Transgenic genome editing is very specific in that only the desired gene is inserted into the host plant genome, which massively reduces the potential for undesired or unintended effects on the plant breeding outcome.”
Although transgenic gene editing has the potential to radically change plant breeding by creating food crops with attributes they wouldn’t normally have—for example, resistance to certain pests or pathogens, higher nutritive value, drought tolerance, and so on—its advancement has been stymied by concerned, and usually misinformed, activist groups and consumers. The technology was developed more than three decades ago, but only eight transgenic crops are commercially available in the United States: alfalfa, corn, cotton, canola, papaya, soybeans, squash, and sugar beets. Moreover, 19 countries in the European Union prohibit the cultivation of transgenic crops.
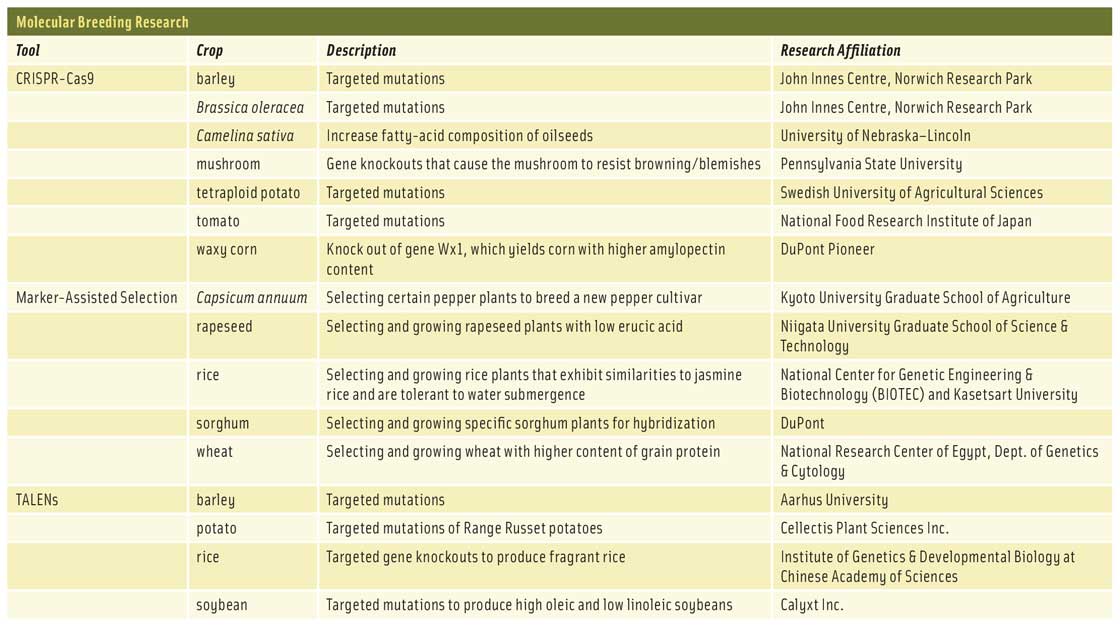
Perhaps the biggest hindrance to universal acceptance of transgenic crops is the fact that transferring genetic material from one species into another incites fierce opposition bolstered by persuasive opinions: To consumers, transgenic gene editing seems unethical, borders on playing God, and creates “frankenfoods” that could cause unintended side effects (e.g., allergies or toxins) in humans and animals. Despite the fact that an abundance of scientific research by biotechnology companies as well as independent researchers not affiliated with the biotechnology industry has determined that genetically modified organisms (GMOs) are safe for consumption, the stigma persists. New genome-editing tools have emerged that may eliminate the basis for consumer concerns: marker-assisted selection (MAS), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPRs). MAS uses DNA markers to more efficiently alter the genetic makeup of domestic crops through selective breeding while TALENs and CRISPRs allow scientists to precisely edit a gene in a species without transferring gene material from another species.
 New Molecular Tools in the Gene-Editing Toolbox
New Molecular Tools in the Gene-Editing Toolbox
With classic breeding techniques, developing improved crop varieties can take more than two decades. This is largely due to the significant reliance on plant phenotypes. MAS is based on plant genotypes, so it can shorten the time needed to develop a new crop by at least 10 years. “Breeding for genotype can be faster, easier, and cheaper when phenotyping plants is difficult and markers for the desired traits are known,” Schultink says. MAS works by locating the genetic traits that distinguish one plant from another, which are encoded in DNA. Thus, any specific trait a plant breeder wants to express in future plant generations is controlled by a gene or group of genes somewhere in the genome of the parent plants. Scientists can find that gene or genes by using a genetic marker. A genetic marker (also called a molecular marker) is a specific segment or sequence of DNA that differentiates one gene from another. For example, 99.9% of the DNA in humans is identical from person to person; less than one percent of human DNA differentiates attributes such as hair color, eye color, disease risk, and so on. A genetic marker may be part of the gene containing a desirable trait, or it may indicate that the desired gene is located nearby. “One example would be if you know a disease resistance trait you want to introgress into an elite variety and you need to do several backcrosses as part of this process. If you have a marker for the desired trait, you can genotype plants when they are young and quickly select the ones you want rather than growing them all and testing them for resistance,” Schultink explains. By mapping genetic markers of a plant’s genome, scientists obtain a better knowledge of where a specific gene is and whether a particular plant variety has it. Plant breeders can then use that particular plant to breed other plants, ensuring that the progeny will have the desired trait. “MAS is used in conjunction with classic breeding techniques to improve trait selection, thus the breeding cycle is shortened to improve the overall process for developing a desired variety,” Wolt points out.
Another genome-editing tool being used to create new crop varieties is TALENs. TALENs are engineered endonucleases that execute double-strand breaks in the DNA structure of a gene. Derived from bacteria, this genome-editing tool requires meticulous selection of the beginning and end points of the sequence to be excised from the DNA double-helix. Once the specific sequences are identified, TALENs can be engineered or customized to cut the target sequences from DNA strands. In essence, “TALENs … can be thought of as molecular scissors that cut DNA at a given site in the genome. [Their] main use is to get rid of unwanted traits, such as susceptibility to disease,” says Jackson. “TALENs are beneficial and complementary to classical breeding.” Scientists use TALENs either to excise a specific DNA sequence (causing a disruption) from a gene and allow the gene to repair itself through mutation (i.e., targeted gene mutagenesis) or to excise a DNA sequence from a gene and insert a different sequence. “It’s possible, though more difficult, to use TALENs to edit the DNA near the cut site to have a desired sequence by supplying an alternate repair template,” Schultink clarifies. Scientists have used TALENs to create disease-resistant wheat and rice and to alter the nutrient content of potato and tomato. None of these crops is commercially available, but Jackson highlights one application that is available: “Recently, TALENs were used to remove the ability of soybeans to make linoleic acid in their oil. The resulting soybean oil is therefore mainly oleic acid,” Jackson says. High-oleic soybean oil is marketed as a replacement for partially hydrogenated oils.
 A newer type of genome-editing system that is also capable of executing double-strand breaks in the DNA structure of a gene is CRISPRs. CRISPRs were identified after scientists observed that bacteria and other prokaryotes had the ability to self-edit their genetic material to improve their immunity to viruses or plasmids. They achieve this by taking a small section of DNA from an invader and splicing that foreign DNA into their own DNA, which turns on CRISPR-associated genes. The bacteria copies the newly formed sequences (i.e., CRISPRs) into ribonucleic acid (RNA), which combines with a CRISPR-associated enzyme (Cas) to detect viral invaders and destroy them. In 2012, two scientists figured out how to modify the CRISPR-Cas system to edit the genomes of other organisms. The process involves programming guide RNA with a specific DNA sequence on an enzyme called Cas9. The RNA guides Cas9 to the target sequence, and Cas9 snips the exact DNA sequence programmed in the RNA. Scientists can then add a DNA sequence at the site of the excision or allow the DNA to repair itself through mutation. The CRISPR-Cas9 system is very precise, so it can edit DNA sequences down to a single letter and conduct multiple edits at once. In addition, while TALENs are like molecular scissors, the CRISPR-Cas system is like a molecular scalpel that is not only more precise but also more efficient and cost-effective. “CRISPR has largely replaced TALENs because it is much easier to use,” Jackson says. This is because “CRISPRs are able to target many more sites for editing within [a] genome than can TALENs,” Wolt adds. Scientists have used CRISPR-Cas to develop new varieties of Brassica oleracea (a species of cabbage), barley, corn, rice, and wheat. Researchers at Pennsylvania State University used the technology to develop a mushroom that resists blemishing (i.e., turning brown) from handling or mechanical harvesting. As with most crops made from TALENs, none of the foods created using CRISPR-Cas is available commercially.
A newer type of genome-editing system that is also capable of executing double-strand breaks in the DNA structure of a gene is CRISPRs. CRISPRs were identified after scientists observed that bacteria and other prokaryotes had the ability to self-edit their genetic material to improve their immunity to viruses or plasmids. They achieve this by taking a small section of DNA from an invader and splicing that foreign DNA into their own DNA, which turns on CRISPR-associated genes. The bacteria copies the newly formed sequences (i.e., CRISPRs) into ribonucleic acid (RNA), which combines with a CRISPR-associated enzyme (Cas) to detect viral invaders and destroy them. In 2012, two scientists figured out how to modify the CRISPR-Cas system to edit the genomes of other organisms. The process involves programming guide RNA with a specific DNA sequence on an enzyme called Cas9. The RNA guides Cas9 to the target sequence, and Cas9 snips the exact DNA sequence programmed in the RNA. Scientists can then add a DNA sequence at the site of the excision or allow the DNA to repair itself through mutation. The CRISPR-Cas9 system is very precise, so it can edit DNA sequences down to a single letter and conduct multiple edits at once. In addition, while TALENs are like molecular scissors, the CRISPR-Cas system is like a molecular scalpel that is not only more precise but also more efficient and cost-effective. “CRISPR has largely replaced TALENs because it is much easier to use,” Jackson says. This is because “CRISPRs are able to target many more sites for editing within [a] genome than can TALENs,” Wolt adds. Scientists have used CRISPR-Cas to develop new varieties of Brassica oleracea (a species of cabbage), barley, corn, rice, and wheat. Researchers at Pennsylvania State University used the technology to develop a mushroom that resists blemishing (i.e., turning brown) from handling or mechanical harvesting. As with most crops made from TALENs, none of the foods created using CRISPR-Cas is available commercially.
The Regulatory Edge
“Genome editing with engineered nucleases—whether using TALENs, CRISPRs, or some other reagent system—provides a powerful approach for discovering gene function and identifying useful traits for plant improvement. Modern plant breeding makes use of a very comprehensive toolbox extending from molecular breeding to classic methods,” Wolt says. So even though TALENs and CRISPR-Cas can be used to breed crops more precisely and quickly, they will never replace classic breeding techniques. “Even with the use of molecular tools, breeding, in the classical sense, is essential. Molecular tools help or assist in this effort, either by improving selection efficiency or by allowing a breeder to select for specific traits, but this is still part of a breeder’s ever-changing and advancing toolbox,” Jackson says. “Most traits we don’t understand the molecular basis for, and even if we did, sometimes traditional breeding is still the best way,” Schultink adds. “In the future, crop breeding will involve many different techniques, including genome-editing methods (like Cas9 and TALENs) and MAS as well as others.”
Still, both TALENs and CRISPR-Cas have the potential to be used to breed crops that are resistant to disease and drought or crops that have reduced levels of allergens or toxins. Unlike transgenic crops, new varieties of crops produced with TALENs and CRISPRs can be created without inserting DNA from other species. “Using genome editing to achieve simple insertions/deletions from [non-homologous end joining] results in products that are indistinguishable from those arising from spontaneous or from chemically induced mutations, which are used in classic plant breeding,” Wolt says. And because such crops are produced through induced mutations, they would not be subject to the U.S. Dept. of Agriculture’s and U.S. Food and Drug Administration’s regulatory requirements for GMOs. In addition, most countries are less wary of mutagenic crops than transgenic crops, so there may be fewer hurdles to approval. Nevertheless, consumers may unilaterally reject crops that are produced by any human-induced genetic modification regardless of whether transferring of DNA is involved. “Current regulations don’t do a good job of matching the degree to which something is regulated with the risk it could pose to consumers and the environment and don’t do a great job of providing clarity in terms of what is allowed and what is not,” Schultink asserts. “This can make it difficult to get beneficial and safe crops to the market. There are many examples from academic research of improvements that can be made to crops to make agriculture more environmentally sustainable and safer but relatively few of these have been able to get through regulation.”
Eradicating the adversities that plants encounter—disease, pests, drought, and nutrient inadequacy—is tied directly to improving human health and well-being by eradicating hunger, famine, and nutrient deficiencies. While genetic modification of crops has been occurring for centuries, the collection of recent genome-editing technologies and tools available to scientists and plant breeders shortens the time necessary to generate crops with newer and better traits. In the quest to feed the world, there should be room for both traditional breeding techniques and genetic engineering. “If we want agriculture to be as safe, productive, and environmentally sustainable as possible, it will be important for breeders to be able to use whatever techniques are most appropriate to achieve a desired outcome,” Schultink says. “I’m optimistic that regulations will be more rational and straightforward in the future to facilitate the use of these techniques to improve agriculture.” Both classic breeding techniques and genetic engineering enable agriculture to provide a highly diverse food supply that meets not only the demands of a changing society but also the demands of a changing climate. For this reason, “it is an exciting time to be a breeder,” Jackson concludes.
Toni Tarver is senior writer/editor of Food Technology magazine ([email protected]).


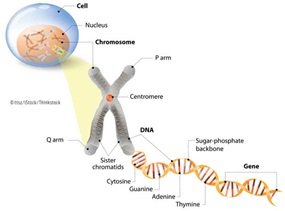 Like humans and other animals, plants are eukaryotes, which means each plant cell contains a nucleus in which linear chromosomes are arranged in pairs. Each species of plant has a set number of chromosomes, and each chromosome carries genes. A gene is the basic unit of heredity in almost all living organisms; it is made up of deoxyribonucleic acid (DNA), a chemical compound that contains the instructions for developing and maintaining an organism. Each DNA molecule consists of two strands made o…
Like humans and other animals, plants are eukaryotes, which means each plant cell contains a nucleus in which linear chromosomes are arranged in pairs. Each species of plant has a set number of chromosomes, and each chromosome carries genes. A gene is the basic unit of heredity in almost all living organisms; it is made up of deoxyribonucleic acid (DNA), a chemical compound that contains the instructions for developing and maintaining an organism. Each DNA molecule consists of two strands made o…



