Bacteriophages - New Weapons for Food Safety
Highly specific phages target and control pathogenic and other harmful bacteria on food products, animal carcasses, and agricultural crops.
Natural bacteriophages or phages are the most-abundant microorganisms in our environment and are present in high numbers in water and foods of various origins. Phages are bacteria-specific—each particular phage is specific for a particular bacterium and is unable to attach to other bacteria. They are harmless to humans, animals, and plants. Over countless millennia, humans have been routinely exposed to phages at high levels through food, water, and the environment, without adverse effect. In certain food products, 100 million phages/g can be found; in aquatic ecosystems, up to 1 billion phages/mL can exist.
 Bacteriophages contribute to bacterial homeostasis in nature, keeping bacteria under control. But thanks to today’s technology, we can now understand and harness this natural tool. Phages will not affect desired bacteria in foods (e.g., starter cultures), commensals in the gastrointestinal tract, and accompanying bacterial flora in the environment. They do not change the organoleptics (i.e. taste, structure, color, and odor) of food products, do not leave an ecological footprint—phages are comprised of and disintegrate into amino acids and nucleic acids, and are normal commensals of humans and animals. This makes them logical agents for the targeted control of certain dangerous bacterial pathogens, such as Listeria in food products. With the emergence of antibiotic resistance, today’s high priority for food safety, and the consumer’s call for “green” and natural products to combat unwanted bacteria, the use of phages may become a new standard.
Bacteriophages contribute to bacterial homeostasis in nature, keeping bacteria under control. But thanks to today’s technology, we can now understand and harness this natural tool. Phages will not affect desired bacteria in foods (e.g., starter cultures), commensals in the gastrointestinal tract, and accompanying bacterial flora in the environment. They do not change the organoleptics (i.e. taste, structure, color, and odor) of food products, do not leave an ecological footprint—phages are comprised of and disintegrate into amino acids and nucleic acids, and are normal commensals of humans and animals. This makes them logical agents for the targeted control of certain dangerous bacterial pathogens, such as Listeria in food products. With the emergence of antibiotic resistance, today’s high priority for food safety, and the consumer’s call for “green” and natural products to combat unwanted bacteria, the use of phages may become a new standard.
Phage Discovery and History
Bacteriophages—Greek for “bacteria eaters”—were discovered nearly a century ago. Edward Twort (1915) and Felix d’Herrelle (1917) independently reported isolating filterable entities capable of destroying bacterial cultures and of producing small cleared areas on bacterial lawns (Figure 1). This implied that discrete particles were involved. They were jointly given credit for the discovery, but it was d’Herrelle who named these entities bacteriophages and who devoted his entire life to researching bacteriophages, including their therapeutic potential.
• Therapeutic applications. Phage therapy was tried extensively, and many successes were reported for a variety of diseases, including dysentery, typhoid and paratyphoid fevers, cholera, and pyogenic urinary tract infections. The early interest is reflected by the fact that some 800 papers on phages were published between 1917 and 1956. The results were quite variable because often uncharacterized phages at unknown concentrations were given to patients without specific bacteriological diagnoses (Ackermann and Dubow, 1987).
With the advent of antibiotics, phage research was abandoned in most of the Western world. Only in France, Poland, and the former Soviet Union was therapeutic phage therapy continued. In France, Jean-François Vieu led the therapeutic phage efforts until the early 1980s. He worked in the “Service des Entérobactéries” of the Pasteur Institute in Paris.
The most-extensive work on phage therapy was carried out under the auspices of the Bacteriophage Institute in Tbilisi in the Republic of Georgia, d’Herrelle’s original institute. In Georgia, phage therapy is still part of the general standard of care, used intensively in pediatrics, burn treatment, and surgical hospital settings.
Worldwide emergence of antibiotic-resistant bacteria has been responsible for renewed interest in phage therapy in the Western world. Improved knowledge of phage ecology, the nature of infections, and improved laboratory methods have resulted in a large number of publications documenting successful phage therapy of experimental infections in animals. Some experiments indicate that phage therapy can be superior to treatment with antibiotics. Much of this work has been reviewed (Levin and Bull, 1996; Barrow and Soothill, 1997). More recent articles on phage therapy cover not only potential treatment of human diseases, but also examination of other applications such as replacing or supplementing antibiotics in aquaculture (Nakai and Park, 2002).
--- PAGE BREAK ---
The cost of clinical trials for new medicines presents a hurdle to phage therapy. However, research provides overwhelming evidence that phages are harmless to humans.
• Natural abundance of phages. The number of phages on the planet exceeds the number of bacteria. Many progeny phages (50-200) are liberated after lysis of the host. This occurs because—in order not to become extinct—at least one of the phages needs to find a new host, often in a three-dimensional matrix before becoming inactivated by factors such as UV light, denaturing, and proteolytic compounds or simple adsorption to particles rendering them inactive.
Bacteriophages are especially abundant in environments with a large number of bacteria. Marine and freshwater ecosystems are teeming with bacteria, and, as a consequence, phage numbers typically reach 107/mL and sometimes exceed this number 300-fold (Fuhrman, 1999; Otawa, et al., 2007; Filippini et al., 2006). Very few foodstuffs are completely sterile. This means that most food consumed will contain bacteria, and, therefore, phages are likely to be present.
This holds especially true for fermented products, as well as for unprocessed vegetables. Phages can readily be isolated from sauerkraut (Yoon et al., 2002; Barrangou et al., 2002). In one study, 26 different phages were isolated from four commercial sauerkraut fermentation plants (Lu et al., 2003).
Phages infecting Propionibacterium freudenreichii have been isolated from Swiss cheese at levels of up to 7 x 105 PFU (plaque-forming units)/g-1 (Gautier et al., 1995). Phages infecting thermophilic lactic acid bacteria have been isolated from Argentinean dairy plant samples at levels of up to 109 PFU/mL (Suarez et al., 2002).
More importantly, non-fermentation culture bacteriophages have also been isolated from various food sources. Escherichia coli phages have been isolated from a large number of products, including fresh chicken, pork, ground beef, mushrooms, lettuce, raw vegetables, chicken pot pie, and delicatessen food, with phage numbers as high as 104/g (Allwood et al., 2004).
Also, Campylobacter phages have been isolated at levels of 4 x 106 PFU/g-1 from chicken (Atterbury et al., 2003), and in another study several different Brocothrix thermosphacta phages were isolated from beef (Greer, 1983).
In all of these cases, the researchers were looking for phages infecting particular bacteria, but when one considers the myriad of bacteria associated with soil and vegetables, it becomes clear that one would also be likely to find phages associated with this multitude of other species.
A recent article on the presence of E. coli and Campylobacter phages in New Zealand vegetables and chicken found coliphages in more than 90% of the samples at numbers of 250/g-1 (Tsuei et al., 2007).
• Evidence of safety. Phages are found in enormous numbers in the environment and in our food. Millions of phages exist in our digestive systems, and we regularly consume millions more with food and water. While this alone provides overwhelming evidence of their safety, additional data corroborate it. Decades of extensive use in medical applications—with exposure to phages not limited to oral uptake, but rather systemic application being common—showed no adverse effects whatsoever. Specific safety-related research has also been performed. An oral toxicity study with rats receiving high doses of Listeria-phage Listex P100 did not reveal side effects (Carlton et al., 2005), and a study in humans, with E. coli-specific phages able to infect commensal E.coli strains as well as pathogenic strains, failed to show any adverse effects (Bruttin and Brussow, 2005).
Two simple safety rules should be kept in mind: temperate phages—easily discernible through genome analysis—should be avoided. Also, phages capable of generalized transduction should be avoided unless the production host is non-pathogenic.
--- PAGE BREAK ---
• Mechanism of action. Bacteriophages do not have their own metabolism, but rely on host bacteria in which to multiply. They exhibit narrow host ranges, usually targeting specific strains. The phage reproduction cycle after host recognition is depicted in Figure 2. The first step is recognition of the host-specific primary receptor, followed by adsorption to the secondary receptor. The first attachment is reversible—the cell might not be alive, and then attachment would be pointless. The second attachment step is irreversible, and subsequently, the phage DNA circularizes, and the bacterium starts producing phage proteins. These proteins sequester the entire host cell and force it to exclusively produce new phages. After assembly of the progeny phages, two separate phage proteins break down the cell envelope (lysis), resulting in release of daughter phages ready to initiate the next cycle. The extremely high specificity of phages for a distinct host is due to each step in the process requiring phage/host systems to be compatible. Being able to recognize cell-wall receptors of a different type is useless if the phage genetic information cannot be “read” by that host. These phage/host systems have evolved over eons to be compatible. Also, every bacterium already has its own complement of specialized phages with which to compete, and a non-specialized newcomer phage would be ineffective. In the absence of target bacteria, the phages break down into common biological particles that are naturally absorbed back into the environment.
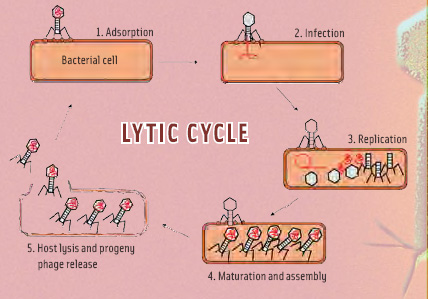
Temperature and host fitness may influence the number of progeny phage and the overall time of the cycle, but for applications in food, it is important to remember that successful infection inevitably leads to cell death. As far as food applications are concerned, in order to remove a contaminating bacterium, it has to “meet” a bacteriophage. If this meeting results in infection, the bacteria will die. In ecological terms, phage populations can only increase when sufficient hosts are present, but this is not the goal in food applications of phages. Here, the goal is eradication of pathogens as the appropriate way to prevent further outgrowth of the pathogens. These will be present only at low numbers under the hygienic conditions encountered in modern food processing, which means that sufficient phages have to be applied for phage/host interaction to become probable. The 50 or so progeny phages resulting from such a meeting will have no statistical influence on the total phage population.
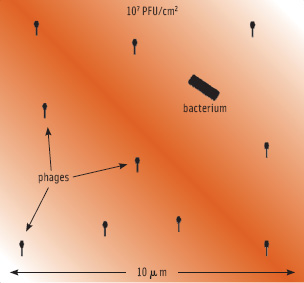
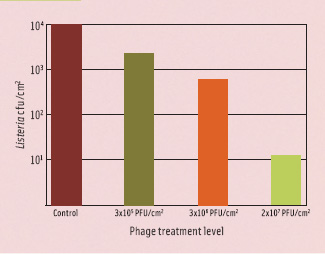
The term MOI (multiplicity of infection) makes sense only when talking about high host numbers and in fluid systems. In food applications, it makes more sense to calculate the phage concentration necessary to infect a single cell on a given surface or in a given volume. This principle is illustrated in Figure 3. It depicts a single cell on a surface treated with 1 x 107 PFU/ cm2. The visible surface in this figure has an area of one millionth of a square cm, so statistically 10 phages are in such an area when using this treatment. Depending on the surface and the amount of fluid deposited, the phages have a limited mobility, allowing them to “search” certain areas for susceptible hosts. The less fluid, the more limited mobility, and the quicker phages will adsorb to the surface and stop moving altogether. Also, phages will be deactivated completely by structural degradation through various ways over time. This means that there will be a certain minimum of phages required to achieve a certain reduction. This is illustrated in Figure 4, showing the effect of different level phage P100 treatment of salmon artificially contaminated with Listeria. At a treatment level of 3 x 105 PFU/ cm2, the phages have virtually no impact, even though they nominally outnumber the bacteria. At a treatment level of 2 x 107 PFU/ cm2, an almost 3 log10 reduction can be observed. At this level of treatment, there is a good chance that a bacterium will be found by a phage.
Bacteriophages in Food Safety
• Target organisms. The food industry is mostly concerned with the “big four” food pathogens: Listeria monocytogenes, Salmonella, Campylobacter, and pathogenic E. coli. Of these, only Listeria regularly colonizes production facilities and thus is able to contaminate food very late in the production process. Therefore, phage treatment at the stage where this contamination is likely to take place is the logical conclusion. For many foods, this will be at some point prior to packaging, but cheese, for example, may be vulnerable to contamination throughout the ripening stage. A number of studies on successful phage treatment of various foodstuffs contaminated with Listeria have been published (Leverentz et al., 2003; Leverentz et al., 2004).
--- PAGE BREAK ---
The other three organisms do not regularly colonize facilities, and it is usually raw products that introduce contamination. As a matter of fact, these organisms colonize animals whose meat is used for human consumption. Therefore, one possible treatment is application of phages during livestock farming, in addition to—or as an alternative to—treating the meat after slaughter. Studies have been undertaken to treat chickens with phages against Salmonella (Fiorentin et al., 2005) and Campylobacter (Atterbury et al., 2007; Wagenaar et al., 2005) and to treat ruminants with phages targeted against pathogenic E. coli (Bach et al., 2003; Raya et al., 2006; Sheng et al., 2006). In most of these studies, significant reductions of bacterial loads were observed. A reduction shortly prior to slaughter can significantly lower subsequent food safety risks and, in any case, does not exclude additional phage treatment at later stages of food production, which several studies show to be highly effective (Leverentz et al., 2001; Whichard et al, 2003). Researchers Goode et al. (2003) report complete eradication of an artificial Campylobacter contamination on chicken skin.
• Environmental application. Treatment of facility surfaces is also a possibility. Food-contact surfaces, in particular, could be cleaned effectively using phages, even during production, without interrupting manufacturing processes. Non-food-contact surfaces could also be treated using phages. However, the added benefit of this seems doubtful because phages cannot reach certain places that aggressive chemicals can. Thus, chemicals, which may readily be used to this end, are likely to be as effective at lower costs.
• Field use. Crop protection is an additional application for phages. Plant diseases caused by bacteria, such as tomato and pepper spot, or fire blight in apple trees, are candidates for phages targeted at the causative pathogens.
Another application is the use of phages for external treatment of an animal prior to slaughter. If, for example, pathogenic E. coli colonizes the intestines of an animal, the bacteria can be found in its excrement, which may be deposited on its hide. Dust from the hide may contaminate the meat after or during slaughter. If, however, the hide is rinsed with a phage solution, this problem may be avoided. Little or no efficacy data has been published in scientific journals on these applications.
• Bacteriophage products and regulatory status. In the United States in the past two years, several bacteriophage applications have been approved for use. In 2006, EBI Food Safety’s Listex, a natural and organic anti-Listeria phage preparation that can be used as a processing aid with all food products susceptible to L. monocytogenes, was approved as GRAS by both the Food and Drug Administration and the U.S. Dept. of Agriculture. In November 2007, Listex was awarded the prestigious Food Ingredients Europe Gold award as best industry innovation. EBI Food Safety, The Netherlands (www.ebifoodsafety.com), viewed as a product leader in phage technology, is also developing phage products against methicillin-resistant Staphylococcus aureus (MRSA), Salmonella, E. coli, and Campylobacter.
In 2007, OmniLytics Inc., Salt Lake City, Utah (www.omnilytics.com), received FDA approval for an anti-E. coli hide wash and an anti-Salmonella wash/mist/spray for treatment of live animals prior to slaughter. In 2006, the U.S. Environmental Protection Agency granted approval to OmniLytics AgriPhage products to combat tomato and pepper spot. The FDA granted approval to Intralytics Inc., Baltimore, Md. (www.intralytics.com), for LMP 102, a cocktail of six different phages to be used as an additive against L. monocytogenes during packaging of poultry and ready-to-eat meat products. An increasing number of companies—and dozens of research groups—are now developing phage technology for many different applications.
Outlook
In the fight against dangerous and unwanted bacteria, phages offer a unique opportunity. The concept of using phages is not new, but we now possess the necessary knowledge to harness their potential. Phages are easy to use and can enhance food safety without affecting the organoleptic properties of foodstuffs, or indeed, without any negative side effects. They offer a completely natural solution to the problem of foodborne pathogens and are set to provide a positive contribution to the food processing industry and public health.
Steven Hagens, Ph.D., ([email protected]) and Mark L. Offerhaus ([email protected]) are, respectively, Chief Scientific Officer and Chief Executive Officer, EBI Food Safety, Nieuwe Kanaal 7P, 6709 PA Wageningen, The Netherlands. Send reprint requests to author Hagens.
References
Ackermann, H.W., and Dubow, M. 1987. Viruses of prokaryotes I: General Properties of Bacteriophages, Chpt. 7 in “Practical Applications of Bacteriophages.” CRC Press Boca Raton, Fla.
Allwood, P. B., Malik, Y.S., Maherchandani, S., Vought, K., Johnson, L.A., Braymen, C., Hedberg, C.W., and Goyal, S.M. 2004. Occurrence of Escherichia coli, noroviruses, and F-specific coliphages in fresh market-ready produce. J. Food. Prot. 67: 2387.
Atterbury, R. J., Dillon, E., Swift, C., Connerton, P.L., Frost, J.A., Dodd, C.E., Rees, C.E., and Connerton, I.F. 2005. Correlation of Campylobacter bacteriophage with reduced presence of hosts in broiler chicken. Appl. Environ. Microbiol. 71: 4885.
Atterbury, R. J., Van Bergen, M.A., Ortiz, F., Lovell, M.A., Harris, J.A., De Boer, A., Wagenaar, J.A., Allen, V.M., and Barrow, P.A. 2007. Bacteriophage therapy to reduce salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73: 4543.
Bach, S.J., McAllister, T.A., Veira, D.M., Gannon, V.P.J., Holley, R.A. 2003. Effect of bacteriophage DC22 on Escherichia coli O157:H7 in an artificial rumen system (Rusitec) and inoculated sheep. Anim. Res. 52:89.
Barrangou, R., Yoon, S.S., Breidt F., Jr., Fleming, H.P., and Klaenhammer, T.R. 2002. Characterization of six Leuconostoc fallax bacteriophages isolated from an industrial sauerkraut fermentation. Appl. Environ. Microbiol. 68: 5452.
Barrow, P. A., and Soothill, J.S. 1997. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 5: 268.
Bruttin, A., and Brussow, H. 2005. Human volunteers receiving Escherichia coliphage T4 orally: a safety test of phage therapy. Antimicrob. Agents. Chemother. 49: 2874.
Carlton, R. M., Noordman, W.H., Biswas, B., de Meester, E.D., and Loessner, M.J. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43: 301.
d‘Herelle, F. 1917. Sur un microbe invisible antagoniste des bacilles dysenteriques. Comptes Rendus de l’Académie des Sciences 165: 373.
Filippini, M., Buesing, N., Bettarel, Y., Sime-Ngando, T., and Gessner, M.O. 2006. Infection paradox: high abundance but low impact of freshwater benthic viruses. Appl. Environ. Microbiol. 72: 4893.
Fiorentin, L., Vieira, N.D., and Barioni, W., Jr. 2005. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in faecal content of broilers. Avian. Pathol. 34: 258.
Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399: 541.
Gautier, M.A., Rouault, A., Sommer, P., and Briandet, R. 1995. Occurrence of Propionibacterium freudenreichii bacteriophages in Swiss cheese. Appl. Environ. Microbiol. 61: 2572.
Goode, D., Allen, V.M., and Barrow, P.A. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69: 5032.
Greer, G.G. 1983. Psychrotrophic Brocothrix thermosphacta bacteriophages isolated from beef. Appl. Environ. Microbiol. 46: 245.
Leverentz, B., Conway, W.S., Alavidze, Z., Janisiewicz, W.J., Fuchs, Y., Camp, M.J., Chighladze, E.. and Sulakvelidze, A. 2001. Examination of bacteriophage as a biocontrol method for salmonella on fresh-cut fruit: a model study. J. Food. Prot. 64:1116.
Leverentz, B., Conway, W.S., Camp, M.J., Janisiewicz, W.J., Abuladze, T., Yang, M., Saftner, R., and Sulakvelidze, A. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519.
Leverentz, B., Conway, W.S., Janisiewicz, W., and Camp, M.J. 2004. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food. Prot. 67: 1682.
Lu, Z., Breidt, F., Plengvidhya, V., and Fleming, H.P. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69: 3192.
Nakai, T., and Park, S.C. 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153:13.
Otawa, K., Lee, S.H., Yamazoe, A., Onuki, M., Satoh, H., and Mino, T. 2007. Abundance, diversity, and dynamics of viruses on microorganisms in activated sludge processes. Microb. Ecol. 53: 143.
Raya, R. R., Varey, P., Oot, R.A., Dyen, M.R., Callaway, T.R., Edrington, T.S., Kutter, E.M., and Brabban, A.D. 2006. Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157:H7 levels in sheep. Appl. Environ. Microbiol. 72: 6405.
Suarez, V. B., Quiberoni, A., Binetti, A.G., and Reinheimer, J.A. 2002. Thermophilic lactic acid bacteria phages isolated from Argentinean dairy industries. J. Food. Prot. 65: 1597.
Tsuei, A. C., Carey-Smith, G.V., Hudson, J.A., Billington, C., and Heinemann, J.A. 2007. Prevalence and numbers of coliphages and Campylobacter jejuni bacteriophages in New Zealand foods. Int. J. Food. Microbiol. 116: 121.
Twort, F.W. 1915. An investigation on the nature of ultramicroscopic viruses. Lancet II: 1241.
Vieu, J.F., Guillermet, F., Minck, R., and Nicolle, P. 1975. Domnees actueles sur les applications therapeutiques des bacteriophages. Bull. d’Acad. Nat. du Med. 163: 61.
Whichard, J. M., Sriranganathan, N., and Pierson, F.W. 2003. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food. Prot. 66: 220.
Yoon, S. S., Barrangou-Poueys, R., Breidt, F. Jr., Klaenhammer, T.R., and Fleming, H.P. 2002. Isolation and characterization of bacteriophages from fermenting sauerkraut. Appl. Environ. Microbiol. 68: 973.
