Food Technology Magazine | Applied Science
Battling Biofilms
In this column, the author describes the stages of biofilm development in food processing plants, methods of removal, and best practices for prevention.

© Artur Plawgo/iStock/Getty Images Plus
One of the old adages of cleaning is that the equipment that has been cleaned should look clean, smell clean, and feel clean. In other words, use your senses when evaluating the efficacy of your cleaning program. It is fairly easy to look at a piece of equipment or a surface to see whether something has been cleaned. If someone walks through a processing facility and sees residual product on a piece of equipment or polymer building up on a fryer, it should be obvious that the operation is not paying enough attention to its cleaning and sanitizing operations. A person’s nose can also be used as a tool for evaluating cleaning. If one smells butyric acid notes while going through a tomato plant, fermentation aromas while going through a fruit plant, or putrid notes emanating from a drain, it is obvious that the sanitation program needs work. The sense of touch may also be used to monitor cleaning efficiency. If one runs their finger over a piece of equipment or food contact surface and it feels slick or “slimy,” there is a problem. The slime may indicate that there is a biofilm concern.
So, what are biofilms? They are layers of bacteria that attach to equipment and other surfaces and to one another with the help of polymeric materials such as proteins or polysaccharides which then trap other bacteria, debris, and other nutrients. Once established, biofilms shed organisms and debris into the process flow. Biofilms were first identified in the mid-1970s (Marriott 1995). Biofilms may form in many different places. Common examples would be the plaque on your teeth or how a damp, shaded deck might become slippery during cool, wet weather or the fouling of the hull of a boat.
Biofilms may be both beneficial and detrimental depending upon where they are found. When producing certain fermented foods, biofilms are essential when it comes to optimizing production. When producing vinegar, acetic acid bacteria (acetobacter) are allowed to grow on wood chips. The biofilm that is formed (Mother of Vinegar) enhances the conversion of the substrate (ethanol) to acetic acid. The detriment occurs when they form on food processing equipment and proceed to contaminate the products. The contamination can cause economic spoilage or spread microorganisms of public health significance such as Listeria monocytogenes. Biofilms have the potential to create other problems, including the adverse effect on heat transfer as they build up; the promotion of corrosion of equipment; fouling of probes that can adversely affect monitoring, and plugging filters, screens, or strainers.
Biofilms may not only pose a public health risk, but they can be devastating to operating efficiencies and significantly increase manufacturing costs. The bottom line is that once the biofilm has formed, it provides the bacteria with a competitive advantage. Biofilm protects the organisms from chemicals, soaps, and other hurdles that we throw at them while it provides the bacteria with a continuous source of food and nutrients. The organisms within the biofilm act synergistically and can be the source of significant and irreparable damage to equipment in addition to the biofilm providing a harborage site for reproduction.
Biofilm Formation
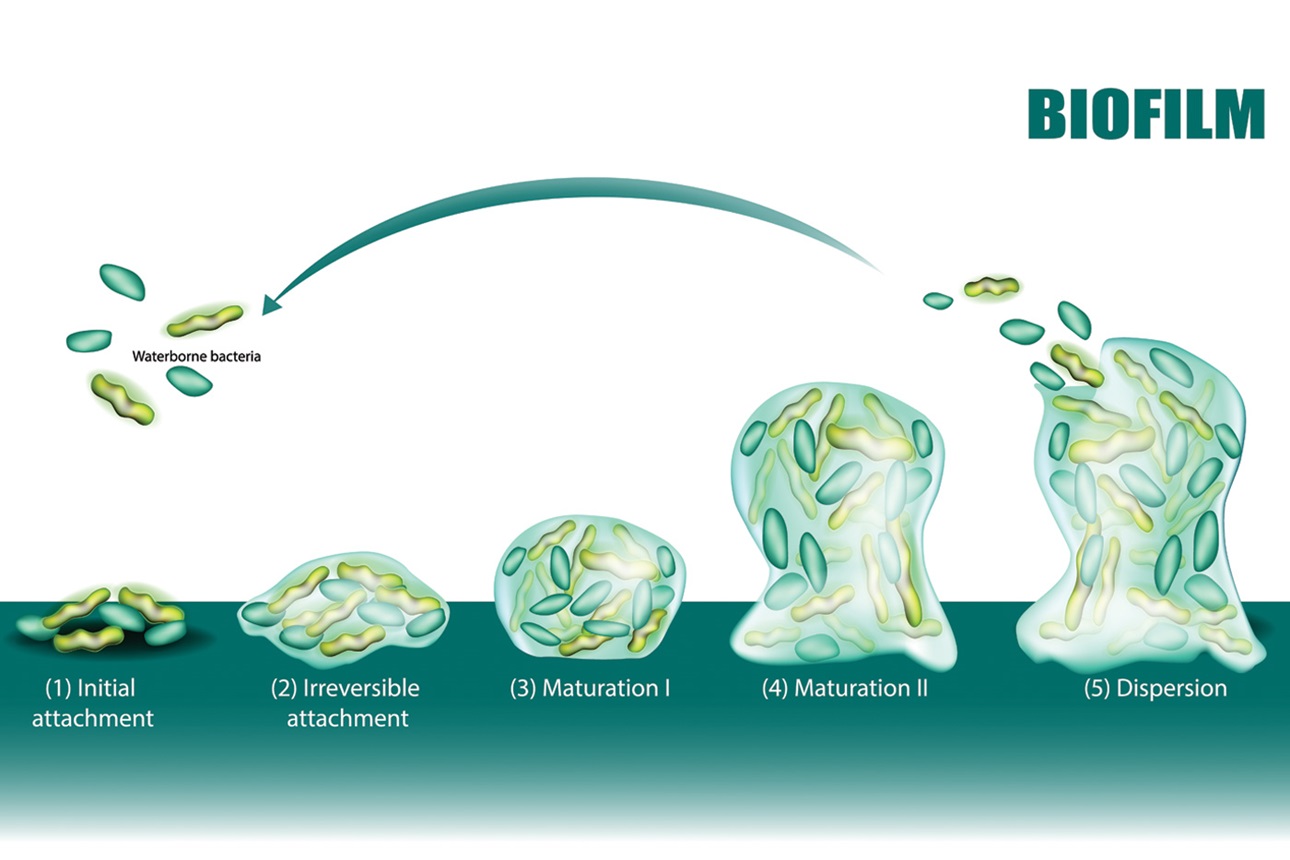
Biofilm formation is not something that occurs suddenly. When one applies a paint or varnish to a surface, it coats the surface, leaving behind a film or coating. If a piece of metal or plastic is dipped in oil, a film of oil remains on the surface. Biofilms need to form over time and in areas where they remain undisturbed. As the biofilm forms and develops, it becomes more complex and the harder it is to remove. This is the reason sanitation or hygiene programs must not only be carried out on a regular schedule but must follow validated cleaning and sanitizing procedures that target the prevention of biofilm formation.
Biofilms have the potential to form on any surface that is exposed to non-sterile liquids or solids. Biofilms can form on all types of surfaces, including stainless steel, compromised gaskets, O-rings, rubber tubing, rusty areas on equipment and in drains, and plastics. The three most common sites are dead ends in pipes, decommissioned equipment left in place and cross-connected to active processing equipment, and in pipes that make sharp angles to move the product. Researchers have shown that biofilms can form on materials such as copper and lead, so a food processor can reasonably assume that biofilms could form anywhere.
They can also form on a wide range of processing operations and products. A great deal of research has focused on the meat, poultry, and dairy processing industries. Why? One reason is that these products provide a rich substrate for growth. In addition, there are many different microorganisms of public health significance associated with these products. Biofilms have also been associated with chilled foods, juice, and juice products, and, most often, in water systems.
There are many different microorganisms that can form a biofilm. These include non-pathogens such Pseudomonas fragi, Enterococcus spp., Pseudomonas flourescens, and Luconostoc spp. along with microorganisms of public health significance such as Salmonella and L. monocytogenes. The fact that certain pathogens can form biofilms is what makes it imperative to understand how and where biofilms can lurk in a processing environment.
The first step in biofilm formation involves the attachment of the microorganisms to the surface. The initial attachment is very weak and involves forces known as van der Waals. At this point in the life and development of a biofilm, removal is easier because the “infant” film only consists of an organic film and the weakly bound bacteria.
During the second phase of biofilm formation, the microorganisms attach themselves to the surface using tendrils or filaments. At the same time, the microorganisms secrete a polysaccharide-like material to cement themselves to the food contact surface and to each other. This polysaccharide or polymer, very similar in its tenacity to super glue, also entraps other microorganisms and debris in the matrix as it forms. Within 24 hours, the microorganisms making up the biofilm are firmly entrenched. In an environment in which there are nutrients that are being continuously supplied to the film, the biofilm develops at a steady rate to the point where the biofilm is both stable and a continuous mass.
Once the surface has been colonized, the film is irreversible and special protocols will be required to fully clean and sanitize the surface. A mature biofilm is an entity that has reached an equilibrium of sorts. The product flowing past the biofilm delivers nutrients, oxygen, and other elements necessary for growth, and carries away byproducts of growth and some of the newly attached cells or other debris that have been sloughed off the surface. Once equilibrium has been reached, the biofilm reaches a certain thickness and remains as such. There are researchers who have broken biofilm formation into the following five phases:
- Transport of nutrients, inorganic, and organic materials to the surface
- Adsorption of conditioning film containing inorganic and organic nutrients
- Attachment of microbial cells to the wetted surface and initiation of growth
- Bacterial metabolism within the film
- Cell disruption and detachment from the biofilm
Biofilm Prevention
Prevention of biofilm formation entails several things. One, and perhaps the most important, is the development, documentation, implementation, and proper maintenance of a good cleaning and sanitizing program. Studies have shown that microorganisms can begin to attach to a surface in a flowing medium as quickly as in 30 minutes, and that a stable biofilm can begin to form within eight hours. If a processor is manufacturing a product that could be prone to biofilm formation, the company should factor that into how frequently cleaning should be done. Longer runs can increase the potential for attachment of microorganisms and the initiation of biofilms formation.
Another weapon for minimizing biofilm formation is the processing equipment. Equipment of poor sanitary design and/or systems that have been improperly installed can be difficult to properly clean. Areas on the line may be inaccessible or hard to clean, hence workers do not take the time or expend the effort to clean the hard-to-reach areas. For example, the installation of oversized pipes or closed lines may be good for moving products, but also may be very difficult to properly clean. If a processor installs a 12-in diameter pipe to move product but fails to install a large enough pump, there will not be sufficient pressure to provide the turbulence necessary to prevent or disrupt the biofilm formation.
Poor maintenance can also enhance the potential for biofilm formation. Damaged lines or badly abraded or scratched surfaces create areas that facilitate the attachment of microorganisms. Sanitation programs that use abrasives and scrub pads, which etch the surfaces of gaskets and O-rings causing micro-harborage sites, must be eliminated. Having a program that not only inspects but also routinely replaces gaskets and O-rings on a set frequency, regardless of how good the items appear visually, will pay for itself in eliminating a major source of biofilms and preventing the organisms from establishing residency in the equipment. Like the finished products that are manufactured, gaskets and O-rings have a shelf life!
Biofilms can also be inhibited through wise use of sanitizers. L. monocytogenes can establish itself in condensate pans below refrigeration systems. Placing slow-release sanitizer blocks in the pans can keep these clean. Similar blocks may also be used in drains to keep them clean and biofilm free but be mindful that some metals will corrode when exposed to certain chemicals. Similar blocks and “socks” can be used in drains to keep them clean and ensure that the drain P traps have biocide continually. Again, be mindful that old cast iron pipes are probably one of the biggest harborage sites for Listeria biofilm in a facility and adding chemicals that cause more rusting will defeat the good intentions. It is essential to know the chemistry of the environment and the chemistry of the biocides and the differences in how each biocide performs. This program requires inspection and vigilance of the chemical and delivery system chosen to ensure that the biocide is still present in a week, two weeks, or three weeks after being put into place.
Removal of Biofilms
The biggest problem with biofilms is that they are exceedingly difficult to remove once they have established themselves. One tool that researchers have adopted to evaluate biofilm formation has been to securely insert steel coupons into processing lines at different locations. The chips are then exposed to the food during operations and the cleaning and sanitizing operations. By using these coupons, researchers have shown that over seven weeks biofilms can form even when exposed to the cleaning chemicals used in the plant.
Biofilms insulate the cells within their mass. They protect the microorganisms within the matrix from chemical cleaners and sanitizers, as well as from scheduled thermal processes prescribed for product safety because the bacteria within the biofilm have greater resistance to heat. It has also been shown that as biofilms age, they become more resistant to cleaners and sanitizers (Mustapha and Liewen 1989, Wirtanen and Mattila-Sandholm 1992).
Research has shown that attached cells can be 150 to 3,000 times more resistant to hypochlorous acid than unattached cells (LeChevallier et al 1992). Another study determined that attached colonies of L. monocytogenes survived exposure to benzalkonium chloride for 12 to 20 minutes, whereas free cells were destroyed within 30 seconds (Frank and Koffi 1992). Scientists who evaluated the effects of a variety of cleaning and sanitizing compounds of biofilms found that it was essential that cleaners and sanitizers be used in combination for more effective removal of biofilms (Krysinski et al 1992). Their work served to affirm an earlier study by researchers who recommended that the proper use of cleaners and sanitizers was essential to properly control biofilms in milk processing (Stone and Zottola 1985).
It is obvious that biofilm removal is not a simple process. The easiest way to deal with a biofilm problem is to be proactive; that is, to have effective programs in place that target the prevention of biofilm formation. A commitment to purchasing equipment of good sanitary design, properly installing that equipment, intelligent production scheduling, and the development, documentation, and implementation of validated cleaning and sanitizing programs using sanitation products that are compatible with the equipment and mechanisms being used for cleaning and sanitizing—along with adherence to these programs—will minimize the potential for biofilm formation.
If a biofilm problem does crop up, it will be necessary to establish a cleaning program that will remove the biofilm. The regular program and the chemicals that were previously used will no longer be effective. Remember, a biofilm consists of soil, microorganisms, and other materials on the surface, as well as a matrix of microorganisms, polymers such as polysaccharides, and soil below the surface. The application of a detergent followed by a rinse will remove the surface soil exposing the subsurface. The theory is that removing the surface will make the subsurface more susceptible to sanitizers. The microorganisms are attached to the equipment surface and embedded in the polymer and so are more resistant than unattached cells and their ability to repel water adds to their survivability. Proper removal of biofilms requires a compound that can penetrate and solubilize the polymers that make up the biofilm and reduce its ability to have surfactant properties (Kramer 1992). The compound that performs best for removing biofilms is a hydrogen peroxide/peroxyacetic acid-based compound.
Biofilms and the Future
Biofilms are not going to go away. In fact, as the food industry moves toward longer processing runs and manufacturing more complex specialty products while facing economic pressures to reduce overall sanitation and chemical costs, the potential for biofilm formation will increase.
The greatest danger with biofilms is that they cannot be removed using regular cleaning and sanitizing protocols. Biofilm protects the organisms, rendering them more heat resistant and having greater resistance to chemicals as LeChevallier’s work strikingly demonstrates. The biofilm will seed product passing over it. If the biofilm was formed by a pathogen, the product will be rendered unsafe. And keep in mind, that it may be shedding the pathogen intermittently making detection of the pathogen in a routine sampling program very randomized and possibly undetectable in the early stages of contamination. If the biofilm was formed by another organism, the result could be product spoilage. Either way, the company will suffer.
The best means for managing biofilms is to ensure that they do not form. Make sure that the equipment adheres to the basic principles of sanitary design; establish and validate and maintain proper procedures for cleaning and sanitizing; establish production scheduling which allows for regular cleaning and maintenance, and utilize the tools that are available like sanitizer blocks to prevent establishment of colonies and new emerging chemicals that are effective against biofilms, such as silver ion-containing chemicals to be used where a persistent biofilm exists or where other chemicals failed to work. Work with the chemical supplier to find the best means to address the issue and discuss the best options for your specific situation, product(s), and equipment. Finally, talk with colleagues in industry as we have all been down the same road previously with different degrees of success.ft