High-Pressure Sterilization: Maximizing the Benefits of Adiabatic Heating
Taking into account the importance of temperature and temperature control could make high-pressure sterilization economically more feasible.
When a product is pressurized, an adiabatic temperature increase always occurs as a result of compression heating. However, most researchers working to develop high-pressure sterilization processes have not taken full advantage of that phenomenon.
This article describes an approach that maximizes the benefits of adiabatic heating and thereby increases enormously the feasibility of high-pressure sterilization of foods. It reports on the importance of temperature control during high-pressure sterilization and describes the development of a new high-pressure sterilization process utilizing a single pressure pulse.
High-Pressure Sterilization
High-pressure processing has emerged as an attractive processing technology for mild preservation of foods. At ambient temperature, pressures in the range of 200–600 MPa reduce the number of microorganisms and inactivate enzymes involved in product spoilage, while the product retains its fresh or just-prepared appearance, organoleptic characteristics, and nutritional quality.
Since the introduction of high-pressure processed products in 1990, the range of products has gradually expanded and now includes jams, jellies, sauces, fruit juices, avocado puree, and ham. The effect that these pressure treatments have on microorganisms is comparable to that of pasteurization, and the products therefore have an extended shelf life under refrigerated conditions.
To achieve a commercially sterile product that can be stored at ambient temperatures, bacterial endospores also need to be inactivated. Two mechanisms have been shown to result in high-pressure inactivation of bacterial spores. At lower pressures and temperatures, pressure induces spores to germinate, and the germinated spores are subsequently inactivated by the pressure treatment (Gould and Sale, 1970; Wuytack et al., 1998). At higher temperatures, a direct spore-inactivation mechanism that bypasses germination is more likely (Maggi et al., 1996; Mills et al., 1998; Ananta et al., 2001). The inactivation curves that are observed under such conditions usually do not follow first-order kinetics and are therefore difficult to interpret (Okazaki et al., 2000; Ananta et al., 2001).
In the patent literature, three different processes have been described for the production of commercially sterile food products:
• Application of a single pressure pulse of at least 700 MPa for 15 min or longer to a product which is first preheated to a temperature above 90°C (Wilson and Baker, 2000, 2001).
• Application of at least two pressure pulses of 700–1,000 MPa to a product which is first preheated to a temperature of 70–90°C (Meyer, 2000, 2001).
• Application of a single pressure pulse of at least 70 MPa for more than 12 hr (Hirsch, 2000).
Lifetime and maintenance costs of high-pressure equipment are related to the number of pulses. Therefore, the processing cost per cycle increases with the number of pulses applied. Processing cost also increases with the total cycle time. Besides reducing the processing costs, a short processing cycle minimizes the effects on the product quality. For a commercial production process, a short production cycle involving a single pressure pulse would be preferred.
Since 1997, our research consortium consisting of Unilever Research Vlaardingen, Stork Food & Dairy Systems, and the Agrotechnological Research Institute ATO has been studying several aspects of high-pressure processing, including design of equipment (Bartels, 1998; Van den Berg et al., 1999); effects on microorganisms and product quality (Krebbers et al., 2002a, b); and packaging and consumer acceptance, with the goal of developing safe, cost-effective high-pressure processes that render high-quality products.
--- PAGE BREAK ---
Adiabatic Temperature Increase
In addition to elevated initial product temperatures, many studies reported in the scientific and patent literature that evaluate high-pressure inactivation of bacterial endospores also exploit the abiabatic temperature increase of the product that is the result of compression heating. Depending on the nature of the product, the initial product temperature, and the applied pressure, the adiabatic temperature increase may vary from 3 to 9°C/100 MPa (Table 1).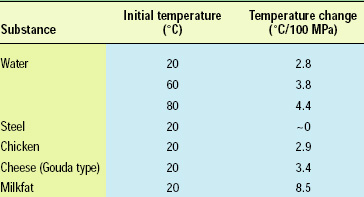
However, while the temperature of a product may thus rise 20–40°C during high-pressure treatment, the metal pressure vessel that surrounds the product is not subjected to significant compression heating (Fig. 1). As a result, the product fraction near the vessel wall cools down and does not reach the same temperature as the product fraction in the center of the vessel (De Heij et al., 2002; Ting et al., 2002).
Many experimental designs for studying the kinetics of inactivation by high-pressure lack measures to control the achieved temperature during treatment (FDA, 2000); this may be the cause of tailing inactivation curves often observed (Ting et al., 2002).
In the experiments that formed the basis for the patented high-pressure sterilization processes at elevated initial temperatures (Meyer, 2000, 2001; Wilson and Baker, 2000, 2001), no measures were taken to prevent heat losses during pressurization. Assuming that the lowest temperature that a product experiences during pressurization determines whether that product is indeed sterilized or not, a similar degree of microbial inactivation may be obtained with lower final temperatures when heat losses to the vessel wall are minimized. Lower final temperatures would allow the process to be operated at a lower initial temperature or a lower working pressure, and would therefore make high-pressure sterilization economically more feasible.
With an axi-symmetric one-dimensional finite element model for heat conduction, it can thus be predicted that in an adiabatic high-pressure sterilization process involving application of a pressure pulse of 700 MPa, the initial product temperature may be lowered from 90°C to 80°C (Table 2) or the pressure may be reduced to 500 MPa.
Technical Solutions
To achieve a cost-effective and commercially feasible, (quasi-) adiabatic high-pressure sterilization process, the following robust, technical solutions may be implemented (Schepdael et al., 2002):
• Apply a high pressurization rate (>5 MPa/sec). For example, fast pressurization can be obtained by using a system with an internal intensifier (Van den Berg et al., 1999). 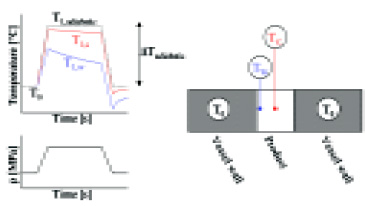
• Apply a vessel material with low heat-transfer properties (less than 1 W/m/°K) and/or with an adiabatic temperature rise that is equal to or greater than the adiabatic temperature rise of the food product (4–8°K/100 MPa). Among the vessel materials suitable for this application are polyoxymethylene (POM), polyetheretherketone (PEEK), or (ultra-high-molecular-weight polyethylene (UHMWPE). These materials can be applied to the interior of the high-pressure vessel as a liner, or the product container can be constructed from these materials.
• Avoid heat losses from the product to the colder pressure fluid entering the pressure vessel. This might be obtained by (a) using an internal intensifier system to generate the pressure, (b) heating the high-pressure pipes and/or the high-pressure pump (external intensifier system), or (c) avoiding thermal contact between the pressure liquid entering the vessel and the product to be treated, e.g., by inserting the product into a product container.
• Avoid heat losses from the product to the pressurization fluid during pressure treatment by choosing a pressurization fluid with an adiabatic temperature rise that is equal to or greater than that of the product.
Whether only one or a combination of these solutions is implemented depends on the improvement in relation to the costs of implementation. In a small-scale laboratory device, application of a product container constructed from, for example, polyethylene or POM with an appropriate wall thickness (>5 mm) will sufficiently control the temperature during treatment.
--- PAGE BREAK ---
Lab-Scale System Tested
A high-pressure system in which all of the above solutions have been implemented has been developed by our consortium for laboratory-scale experiments (Fig. 2) and used to study the kinetics of inactivation of Bacillus stearothermophilus spores. This organism was chosen as a target organism because its spores are among the most heat resistant known.
For production of spores, B. stearothermophilus strain ATCC 7953 was cultivated at 55°C on Spo8 medium (Faille et al., 1999). After 7 days of incubation, spores were harvested, washed in demineralized water, and stored at 7°C. Spores produced according to this procedure were characterized by a thermal decimal reduction time D120 of 8.8–9.9 min and a zvalue of 6.5–6.7°C.
For high-pressure treatments, spores were suspended in tryptone soy broth to a final density of 106–107 spores/mL. Aliquots of this suspension were transferred to polyethylene pouches and stored on ice until processing. Prior to high-pressure treatment, the spore suspensions were preheated for 1 min at the desired initial product temperature. Spores in the untreated spore suspensions and surviving spores in the treated pouches were enumerated by pour plating in tryptone soy agar.
From the observed reductions in viable count, the reaction rate k was calculated as function of final pressure and final temperature. A total of 29 k-values corresponding to various combinations of temperature (84–122°C) and pressure (300–800 MPa) were obtained and fitted with the modified Eyring-Arrhenius equation:
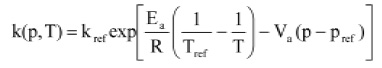 where k and kref are in sec-1, T and Tref are temperature in oK, and p and pref are pressure in MPa. The data fit this first-order kinetic equation with an explained part of 99%.
where k and kref are in sec-1, T and Tref are temperature in oK, and p and pref are pressure in MPa. The data fit this first-order kinetic equation with an explained part of 99%.
Fig. 3 shows the dependency of k and D as a function of p for several final product temperatures. From this graph, it can be concluded that over the range of 300–800 MPa, the degree of spore inactivation is largely determined by the final product temperature and to a relatively small extent to pressure.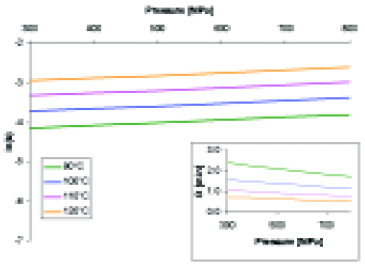
For example, an isothermal (T1 = 100°C) increase from 300 MPa to 800 MPa yields an inactivation rate similar to that of an isobaric (p = 300 MPa) increase from 100°C to 109°C. A similar pattern was observed by Ananta et al. (2001), who evaluated both a first- and a 2.5-order kinetic reaction to calculate the kvalues. The importance of the final product temperature for the degree of spore inactivation confirms the need for measures for controlling the temperature during high-pressure sterilization.
It has been suggested that high-pressure sterilization requires at least two pressure pulses because a single pressure pulse would only sublethally injure cells (Meyer, 2000, 2001). This suggestion was based on the observation that no surviving spores were present immediately after treatment whereas growth had occurred in similarly treated samples after one week of storage.
We evaluated this phenomenon using spores of Bacillus subtilis. In contrast to B. stearothermophilus, this species is able to grow both aerobically and anaerobically at ambient temperatures. Serially 10-fold-diluted spore suspensions of B. subtilis strain 168 were prepared in growth medium consisting of peptone and sodium chloride and transferred to polyethylene pouches. Some of the pouches were used immediately after treatment to determine the viable count by plate counting in tryptone soy agar. The remaining pouches were stored at 37°C for 30 days, then were visually evaluated for growth.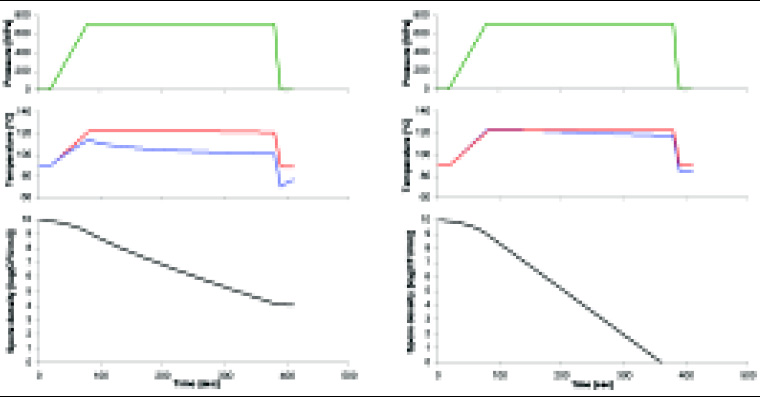
--- PAGE BREAK ---
From the pattern of occurrence and absence of growth in the pouches (2–3 replicates per spore concentration), the number of surviving spores was estimated by the most probable number (MPN) method, a statistical approach for the enumeration of microorganisms in serial dilutions, according to Food and Drug Administration guidelines. The MPN of surviving spores after 30 days of storage did not differ significantly from the plate counts immediately after treatment, so there was no indication of recovery of spores during storage.
Prediction Model Developed
Based on the insights presented above, we have developed a simulation model that predicts the degree of high-pressure spore inactivation as a function of process parameters, equipment material and dimensions, product characteristics, and target microorganism in a two-step process:
1. Calculate the temperature distribution inside the vessel during processing, using an axi-symmetric one-dimensional finite element model based on heat conduction.
2. Then calculate spore inactivation as a function of time and the realized product temperature and pressure, using the Eyring-Arrhenius equation presented above.
Fig. 4 presents two examples in which the effect of the application of an insulating product container in a standard steel vessel on spore inactivation is demonstrated. The high-pressure treatment consisted of a single high pulse of 700 MPa with a hold time of 3 min applied to a product preheated to an initial temperature of 90°C and containing 1010 spores of B. stearothermophilus/mL. In a standard steel vessel, such a process cycle would result in a 6-log spore inactivation, whereas use of the product container would result in a spore inactivation of more than 10 log units.
This predictive model for high-pressure spore inactivation is a useful tool for optimizing high-pressure sterilization processes. In combination with a database of kinetic inactivation parameters of relevant target microorganisms such as Clostridium botulinum, the model may also assist in the validation of high-pressure sterilization processes (Sizer et al., 2002).
Increased Use Foreseen
As discussed above and shown in Fig. 5, compared to a conventional retort process, high-pressure sterilization involves a smaller time–temperature integral as a result of the instantaneous adiabatic temperature rise, and a lower maximum temperature as a result of the additional pressure. Therefore, high-pressure sterilization allows the production of shelf-stable products while minimizing the adverse effects of conventional heating processes on the sensory and nutritional quality. Use of the proposed technical solutions to maximize the benefits of adiabatic heating and the prediction model is expected to lead to faster implementation and greater utilization of high-pressure sterilization in the future.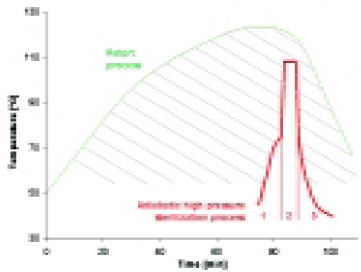
Wouter B.C. de Heij, Ludo J.M.M. van Schepdael, Roy Moezelaar, Hans Hoogland, Ariette M. Matser, and Robert W. van den Berg
Authors De Heij, Moezelaar, and Matser are, respectively, Senior Scientists in Process Engineering, Microbiology, and Food Technology, and author Van den Berg is Head of the Dept. of Preservation Technology & Food Safety, Agrotechnological Research Institute (ATO b.v.), P.O. Box 17, 6700 AA Wageningen, The Netherlands. Author Van Schepdael is President, Solico, Everdenberg 97, 4902 TT Oosterhout, The Netherlands. Author Hoogland is Senior Technologist, Mechanical Engineering, Unilever Research Vlaardingen, P.O. Box 114, 3130 AC Vlaardingen, The Netherlands. Send reprint requests to author Moezelaar.
This study was financially supported by the Dutch Dept. of Economic Affairs program EETA97033. The authors thank Huub L.M. Lelieveld of Unilever Research Vlaardingen for stimulating discussions.
References
Ananta, E., Heinz, V., Schlüter, O., and Knorr, D. 2001. Kinetic studies on high-pressure inactivation of Bacillus stearothermophilus spores suspended in food matrices. Innovative Food Sciences and Emerging Technologies. 2: 261-272.
Bartels, P.V. 1998. High pressure reactor. European patent 0842696 A1.
De Heij, W., Van Schepdael, L., Van den Berg, R., and Bartels, P. 2002. Increasing preservation efficiency and product quality through control of temperature distribution in high pressure applications. High Pressure Res. 22: 653-657.
Faille, C., Fontaine, F., and Membré, J.-M. 1999. Factors influencing recovery of heat-injured Bacillus thuringiensis spores. Statistical approach. J. Food Sci. 64: 363-366.
FDA. 2000. Kinetics of microbial inactivation for alternative food processing technologies—high pressure processing. Center for Food Safety and Applied Nutrition, Food and Drug Admin., Washington, D.C. http://vm.cfsan.fda.gov.
Gould, G.W., and Sale, A.J.H. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60: 335-346.
Hirsch, G.P. 2000. Hydraulic pressure sterilization and preservation of foodstuff and feedstuff. U.S. patent 6,033,701.
Krebbers, B., Matser, A.M., Koets, M., Bartels, P.V., and Van den Berg, R.W. 2002a. High pressure-temperature processing as an alternative for preserving basil. High Pressure Res. 22: 711-714.
Krebbers, B., Matser, A.M., Koets, M., and Van den Berg, R.W. 2002b. Quality and storage-stability of high-pressure preserved green beans. J. Food Eng. 54: 27-33.
Maggi, A., Gola, S., Rovere, P., Miglioli, L., Dall’Aglio, G., and Lonneborg, N.G. 1996. Effects of combined high pressure-temperature treatments on Clostridium sporogenes spores in liquid media. Industria Conserve. 71: 8-14.
Meyer, R.S. 2000. Ultra high pressure, high temperature food preservation process. U.S. patent 6,017,572.
Meyer, R.S. 2001. Ultra high pressure, high temperature food preservation process. U.S. patent 6,177,115 B1.
Mills, G., Earnshaw, R., and Patterson, M.F. 1998. Effects of high hydrostatic pressure on Clostridium sporogenes spores. Lett. Appl. Microbiol. 26: 227-230.
Okazaki, T., Kakugawa, K., Yoneda, T., and Suzuki, K. 2000. Inactivation behaviour of heat-resistant bacterial spores by thermal treatments combined with high hydrostatic pressure. Food Sci. Technol. Res. 6: 204-207.
Schepdael, L.J.M.M., De Heij, W.B.C., and Hoogland, H. 2002. Method for high-pressure preservation. PCT patent application WO 02/45528 A1.
Sizer, C.E., Balasubramaniam, V.M., and Ting, E. 2002. Validating high-pressure processes. Food Technol. 56(2): 36-42.
Ting, E., Balasubramaniam, V.M., and Raghubeer, E. 2002. Determining thermal effects in high-pressure processing. Food Technol. 56(2): 31-35.
Van den Berg, R.W., Bartels, P.V., and Van Schepdael, L.J.M.M. 1999. High-pressure apparatus. PCT patent WO 99/61146.
Wilson, M.J., and Baker, R. 2000. High temperature/ultra-high pressure sterilization of foods. U.S. patent 6,086,936.
Wilson, M.J., and Baker, R. 2001. High temperature/ultra-high pressure sterilization of foods. U.S. patent 6,207,215 B1.
Wuytack, E.Y., Boven, S., and Michiels, C.W. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64: 3220-3224.
