Use of Botanicals as Biopreservatives in Foods
Natural products from plants are finding increased use as antimicrobials in foods, requiring reliable information on their safety and efficacy.
Biopreservatives—a wide range of natural products from both plants and microorganisms—can be useful in extending shelf life of foods, reducing or eliminating survival of pathogenic bacteria, and increasing overall quality of food products. As the popularity of botanical biopreservatives continues to increase, consumers, regulatory agencies, and food processors require reliable information on the safety, standardization, and efficacy of these products.

Regulation
Throughout recorded history, spices and herbs have been used for flavoring foods and beverages and for medicinal purposes. Historical records show the use of botanicals for flavor and medication as early as 6000 B.C. in China. Roman history records that Alarich, a leader of the Goths, laid seige to Rome in A.D. 408 and demanded as ransom various precious metals and 3,000 lb of pepper (Scheiper, 1993). The possession and use of spices and herbs has historically been associated with wealth and prosperity, and today’s economic climate is no different. Black pepper is still the most heavily used spice for flavoring foods throughout the world.
The value of functional foods was $11.3 billion in 1995, $16.2 billion in 1999, and $27 billion in 2003 and is projected to grow to $49 billion in 2010 (Blumenthal, 2003; Hughes, 2000). The term functional foods often includes botanicals that are used as either dietary supplements or food additives. The rapid growth of this market has resulted in major changes in regulatory oversight of botanical ingredients by the Food and Drug Administration.
Dietary supplements are regulated under the 1994 Dietary Supplement Health and Education Act (DSHEA). This new law amended the Federal Food, Drug, and Cosmetic Act and created a regulatory frame-work for the safety and labeling of dietary supplements, including vitamins, minerals, herbs, botanicals, amino acids, specific enzymes, concentrates, metabolites, and extracts. Some companies label foods that are normally considered to be foods (e.g., some drinks) as dietary supplements to avoid preparing a Generally Recognized as Safe (GRAS) affirmation notice. Under DSHEA, a botanical is exempted from the food additive category, and GRAS submission of safety evidence is not required as long as that ingredient was on the market before October 1994.
On January 30, 2001, FDA issued a letter to manufacturers regarding botanical and other novel ingredients in conventional foods (Lewis, 2001). The letter pointed out that there had been a significant growth in the marketplace of conventional food products that contain novel ingredients, such as added botanical ingredients or their extracts, that had not previously been used as food ingredients. The letter reminded manufacturers that many ingredients intentionally added to a conventional food are food additives and not supplements.
A botanical or herb used as a food additive must undergo premarket approval; i.e., the manufacturer must submit a GRAS affirmation notice based on data demonstrating safety to FDA’s Office of Premarket Approval (FDA, 1997). A substance is exempt from the definition of a food additive, and thus exempt from premarket approval, if it is generally recognized as safe by qualified experts under the conditions of intended use (FDA, 2003a). A problem sometimes arises with interpretation of the conditions of intended use, since spices and other botanicals are generally used as flavors at fairly low levels in foods. When botanicals are used as biopreservatives, higher levels are generally required than would normally be used for flavoring. The use of higher levels of botanicals above the amounts normally used for flavor intended use) will then trigger the requirement for submission of a GRAS notice to FDA.
A practical consequence of the toxicology axiom “The dose makes the poison” is that a substance that is safely consumed in the diet at low levels may be unsafe if consumed at a higher level in the diet. Therefore, a second requirement for GRAS affirmation of botanicals is that data must be provided from the literature or from other sources demonstrating that the botanical has been shown to be safe when consumed at the higher level anticipated in the diet.
--- PAGE BREAK ---
Common causes for rejection of GRAS notices for premarket approval of botanical products include the following: (1) dietary exposure to the product was not estimated, (2) no specific manufacturing conditions were provided other than saying that Good Manufacturing Practices will be followed, (3) there were no specifications to describe a food-grade material, (4) clinical studies cited were for a different preparation of the product with different specifications, (5) the no Common causes for rejection of GRAS notices for premarket approval of botanical products include the following: (1) dietary exposure to the product was not estimated, (2) no specific manufacturing conditions were provided other than saying that Good Manufacturing Practices will be followed, (3) there were no specifications to describe a food-grade material, (4) clinical studies cited were for a different preparation of the product with different specifications, (5) the notice did not provide a sufficient basis for determination that the product is GRAS under the conditions of intended use, (6) the addition of the product violates the standards of identity for the proposed food product, (7) unpublished studies which are not generally available to the expert scientific community cannot provide a basis for GRAS determination of the product, (8) the notice did not provide a substantial history of consumption for food use of the product, (9) the reference provided pointed out that the product should only be used occasionally, which undermines the claim that the product is GRAS for the intended use, and (10) active ingredients in the product have been shown to be mutagenic and carcinogenic at the level of intended use (FDA, 2003b).
Any food containing a botanical which is not GRAS causes the food to be adulterated and the food cannot be legally imported to or marketed in the United States. FDA has taken a firm stance on botanical food additives and has issued a number of proposed rules, final rules, warning letters, fact sheets, and directives to assist manufacturers in producing safe and appropriately labeled food products.
As interest in using botanicals as biopreservatives has increased in the U.S., sales of botanicals for use as nutritional supplements has decreased in both the U.S. (down 13% in2003) and some European countries such as Germany (Blumenthal, 2003). The three major losers in the U.S. markets in 2003 were kava kava, ginseng, and St. John’s wort (down 53%, 31%, and 37%), respectively. A variety of reasons can be found for the reduction in sales. However, major factors affecting the market loss are health concerns associated with consumption of these specific botanicals and conflicting data on their efficacy in clinical trials.
The U.S. is not alone in taking a tougher stance on food supplements/additives. On July 3, 2003, the European Union Food Supplements Directive was passed into English law. On July 31, 2003, the Directive passed into the national legislature of the 15 EU member countries. This law will affect approximately 5,000 products presently in European markets (Verkerk, 2003).
Standardization
For labeling purposes, the International Code of Botanical Nomenclature St. Louis Code (Greuter et al., 2000, also available at www.bgbm.fu-berlin.de/iapt/nomenclature/code/SaintLouis/0000St.Luistitle.htm) establishes the current internationally accepted rules that govern the scientific naming of plants. The standardized common name of each botanical for labeling can be found in bold letters in the publication Herbs of Commerce, 2nd ed. (McGuffin et al., 2000). These publications are specifically referenced in FDA’s proposed rule for food labeling of dietary supplements containing botanicals (FDA, 2003c, d). Additional guidelines, definitions, and explanations concerning the use of spices, herbs, and other botanical ingredients can be found in the Code of Federal Regulations (FDA, 2003e).
Crude and partially purified botanical extracts are commonly manufactured to contain a defined amount of a particular compound, constituent, or group of constituents. The particular compound or constituent is called a “marker compound.” One important aspect of standardization of botanical ingredients is to ensure that batch-to-batch variation of the marker compound is within acceptable limits. Although this is the way in which botanical products have been standardized in the past, this does not actually equate to a standardized product.
A study on antimicrobial activity of furocoumarins from parsley reported that chemical composition of parsley varied significantly (P < 0.01) in four varieties of Petroselinum crispum grown in Minnesota (Manderfeld et al., 1997). Psoralen content of parsley varied from 1.94 to 52.72 µg/g dried parsley, and 5-methoxypsoralen ranged from 19.89 to 459.20 µg/g dried parsley. Since the minimum inhibitory concentration (MIC) of psoralen, 5-methoxypsoralen, and 8-methoxypsoralen ranged from 3.5 to 101 ng/g for Listeria monocytogenes, variations in furocourmarin composition of parsley would have serious implications if this botanical were used as an antimicrobial.
--- PAGE BREAK ---
An extract, dried preparation, or essential oil from a plant may also contain hundreds of other chemicals which can affect quality and standardization of the extract (Bisset, 1994). An evaluation of commercial products by the American Botanical Council Ginseng Evaluation program showed that retail root powder products had >30% standard deviation in the marker compound when five lots of the same product were purchased and tested. The standard deviation of the marker compound for extracts of the botanical product (N = 5) ranged from 6% to 87.5% depending on the manufacturer (Hall et al., 2001). This lack of uniformity in composition of botanical ingredients is not particularly surprising, since marker compounds in botanical products are affected not only by the variety of plant but also by the geographic origin, part of the plant which is used, age and growth conditions of the plant, method of extraction or drying, preparation, packaging, and storage.
A comprehensive and informative guidance document on the use of botanicals in food products was developed by a working group of the Natural Toxin Task Force of the European Branch of the International Life Sciences Institute (ILSI)-Europe and discussed with scientists at a conference held on May 13-15, 2002, in Marseilles, France (Schilter et al., 2003). This guidance document stresses that it is essential that botanical ingredients which will be used in food products be well identified and characterized. There are three major aspects which must be addressed to ensure that botanical materials are consistent: (1) the starting material must be accurately identified, (2) the method of preparation must meet good manufacturing practices, and (3) the final product must be standardized (Schilter et al., 2003).
Identification of the plant should include the scientific name and common name, the part of the plant used, harvest date, specific geographic origin, and clear information on the producer and chain of custody for the plant, including contact information for use in an emergency. If a patented variety or other specialized plant is used, this should be noted. Since growth conditions strongly influence the amount and type of bioactive constitutents in the plant, the growth conditions for each lot should be carefully documented. This would include site of collection, time of harvest, approximate age of plant, cultivation practices (note if wild type), pesticide use, standardized agricultural practices followed by the producer, handling and drying practices, storage conditions and type of storage facilities, and decontamination practices (including irradiation).
A standard protocol should be developed for testing each incoming batch of raw material to ensure that composition of the botanical meets company specifications. Quantitative analysis should focus not only on the marker compound but also on other constituents of the botanical which may affect overall activity and acceptability of the preparation and on compounds most likely to be associated with toxicity.
Detailed information on the manufacturing process, including a flow diagram, should be developed with special attention to activities and environmental factors that may affect stability, activity and quality of the botanical preparation (FDA, 2003c, e). Manufacturing botanical preservatives using good manufacturing practices with clearly defined manufacturing activities and specifications will permit the food industry to effectively utilize botanical preparations as biopreservatives to produce acceptable food products.
Companies purchasing botanical preparations for use in their product as biopreservatives should set very clear specifications which permit minimal variation in the batch-to-batch levels of active ingredients. Specifications should include information on the specific level of major marker compounds, type of preparation, concentration equivalent, storage conditions, level of toxic or biologically active compounds, assurance that the product is pure and does not contain harmful levels of mycotoxins, microorganisms, residual solvent, pesticides, heavy metals, dioxin, or other environmental contaminants. Since many of the highly antimicrobial botanical preparations are essential oils, volatility and stability in the final product should be routinely monitored by the manufacturer to ensure the highest-quality product.
Efficacy and Applications
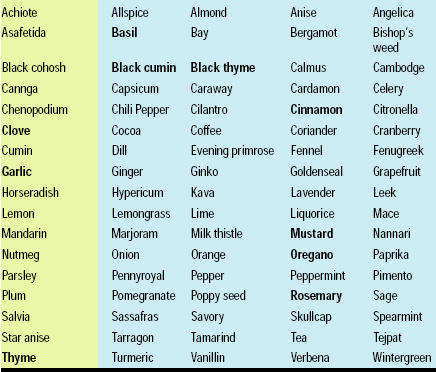
It is estimated that there are 250,000–500,000 species of plants on Earth (Borris, 1996). Approximately 1–10% of these are used as food and medicinals by humans or other animals. Thousands of compounds have been isolated from plants which are claimed to have antimicrobial or medicinal properties (Schultes, 1978). Several hundred research papers are published each year on the antimicrobial activity or other functional activity of botanicals, and a complete review of that work is beyond the scope of this article. There are numerous excellent reviews which discuss this work (e.g., Conner, 1993; Cowan, 1999). Table 1 provides a list of the botanicals which have been shown to have antimicrobial activity.
--- PAGE BREAK ---
Antimicrobial botanicals which have the potential to be used as biopreservatives can be divided into several useful categories, including phenolics, polyphenols, quinones, flavones, flavonoids, flavonols, tannins, coumarins, terpenoids, alkaloids, lectins, and polypeptides (Cowan, 1999). Many herbs, such as thyme, contain multiple active compounds which represent different chemical families. The essential oil (quinta essentia) fraction of botanicals is often the most inhibitory chemical fraction to growth and survival of microorganisms. Essential oils are highly enriched with terpenoids. Examples of herbs or spices containing terpenoids which have been shown to have antimicrobial activity include allspice, basil, bay, burdock, cinnamon, paprika, chili pepper, clove, dill, eucalyptus, gotu kola, grapefruit seed extract, horseradish, lemon verbena, oregano, pao d’arco, papaya, peppermint, rosemary, savory, sweet flag, tansy, tarragon, thyme, turmeric, valerian, and willow (Duke, 1985; Cate, 2000). The other major chemical group found in plants which has been frequently reported to have antimicrobial activity is the sulfoxide/isothiocyanate family, which includes onion, garlic, mustard, and members of the Brassica family. Approximately 30% of essential oils which have been examined are inhibitory to bacteria, and more than 60% of essential oil derivatives have been shown to be inhibitory to fungi (Chaurasi and Vyas, 1977; Cowan, 1999).
The mechanism of action for the antimicrobial activity of botanical biopreservatives is not fully understood. However, terpenoids and phenolics are thought to exert inhibitory action against microorganisms by membrane disruption (Cichewicz and Thorpe, 1996; Lambert et al., 2001; Schultes, 1978). Simple phenols and flavonoids appear to inhibit growth by binding to biochemicals essential for metabolism (Peres et al., 1997). Both coumarins and alkaloids are thought to inhibit growth of microorganisms at the genetic level (Hoult and Paya, 1996; Rahman and Choudhary, 1995).
Although numerous studies have been done in vitro to evaluate the antimicrobial activity of botanicals, only a few studies have been done with food products. Inhibition of fungal growth on bread was reported using allyl isothiocyanate (AITC), which was applied in active packaging (Nielson and Rios, 2000). The sensory threshold was slightly higher than the MIC for AITC on rye bread but lower on hot dog buns. A recent study by Suhr and Nielsen (2003) with rye bread showed that thyme, clove, and cinnamon inhibited spoilage fungi, while orange, sage, and rosemary oils had very limited effects. Mustard and lemongrass essential oils were most effective for volatile application, while phenolics and terpenoids were more inhibitory when applied to the product. Cinnamon, clove, and cardamom oil were found to suppress growth of microorganisms in cookies; however, cardamom reduced overall sensory and quality attributes (Adel et al., 2002).
Black cumin essential oil (ethanolic extract) applied in a teriyaki marinade base to raw trout was found to reduce aerobic plate counts, yeasts and molds, and coliform counts by >log 3.0 cfu/g. L. monocytogenes was reduced to nondetectable levels after 9 days of storage at 4 or 0°C (Elgayyar and Draughon, 1999).
A lemon-based Italian-style marinade containing a combination of 0.3% oregano and 0.3% thyme essential oils significantly reduced aerobic plate counts and Escherichia coli O157:H7 by log 3 cfu/g (P < 0.05) on raw chicken breast fillets. The combination of oregano and thyme (0.3% each) was highly lethal (~ log 4 cfu/g reduction) to Salmonella Typhimurium, Campylobacter jejuni, and L. monocytogenes on marinated chicken breast stored at 4°C (Cate et al., 2000). Tsigarida et al. (2000) found that 0.8% oregano essential oil reduced aerobic bacterial counts, lactic acid bacteria, and L. monocytogenes in meat stored at 5°C regardless of the atmosphere under which the meat was stored.
Marinades containing pimento leaf essential oil (6%) reduced Arcobacter butzleri growth by log 2 cfu/cm2 on pork loins by day 5 of storage (Hancock and Harrison, 2002). Several botanical preparations have been tested in meat products. Dried plum puree (3%) was found to reduce E. coli and Salmonella in ground meat (Pszczola, 2002). A mixture of 2.4% paprika, 0.12% oregano, and 0.05% garlic was found to stimulate the growth rate of Penicillium spp. in Spanish fermented sausage (Diaz et al., 2002). In an acidified chicken meat model, 0.1% oil of mustard was found to have little or no effect on microbial growth after 2 days of storage at 22 °C (Lemay et al., 2002). Cilantro oil was evaluated in vacuum-packaged ham using a surface application of 6% but was found to be ineffective under the conditions of the study (Gill et al., 2002).
--- PAGE BREAK ---
Freshly ground garlic when added to mayonnaise at a concentration of 1% reduced Salmonella Enteritidis counts from log 5 cfu/g to <log 2 cfu/g (too low to enumerate) after 96 hr at 25°C. In controls, Salmonella was also reduced but remained at levels between log 2 and log 3 cfu/g after 96 hr at 25°C (Leuschner and Zamparini, 2002). Garlic has also been shown to reduce levels of E. coli O157:H7 in ground meat (Ceylan et al., 1998) and cinnamon has been shown to reduce levels of E. coli O157:H7 in apple juice (Ceylan et al., 1999).
Lemon, mustard, cumin, and pepper were added to a fish sauce (mehiawah). Although lemon and pepper had no effect, mustard was highly effective in controlling E. coli in the fish sauce, which was stored at 25°C for 28 days (Al-Jedah et al., 2000).
These studies shows that some botanicals have the potential to be effective biopreservatives, although product development to optimize functionality and flavor will be challenging. More studies are needed on applications of botanical preservatives in foods to fully understand how best to optimize their use and to provide basic research to support the food industry.
Safety
Most of the botanical biopreservatives which might be used in foods have been consumed safely by humans for thousands of years. However, it is virtually impossible to find the typical toxicological information such as Acceptable Daily Intake (ADI) or No Effect Level (NOEL) that would be calculated for a new food additive. It may not even be appropriate to expect such calculations for botanical antimicrobials, since the physiological activity of the plant materials can be quite high and the margin of safety between typical use and toxicity levels rather small.
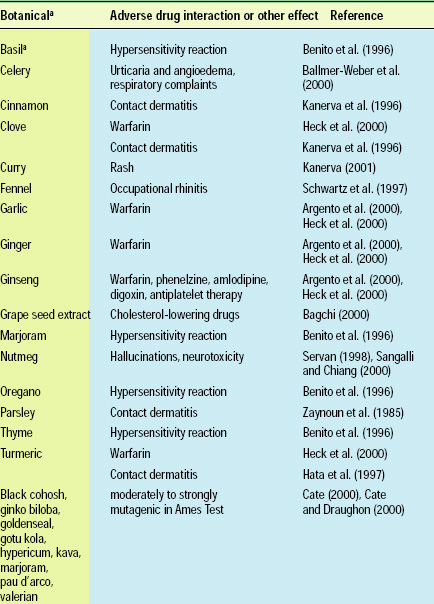
Other factors may also affect the use of botanical biopreservatives by food manufacturers, such as unusual sensitivities of some parts of the population to specific herbal compounds or strong aromatic ingredients. Interaction of some botanical preservatives with prescription and over-the-counter medications has been demonstrated in several scientific studies. Some important interactions, sensitivities, and toxicities are summarized in Table 2.
The key to using botanical preservatives safely is to conduct a standard risk assessment which will identify the hazards that might be encountered with a product. Any hazards associated with use of the botanical will need to be carefully investigated and characterized using the literature, epidemiological studies, clinical data, demographic information, pharmacological studies, and experimental human or animal studies. It is essential to calculate the average daily intake to ensure that no negative nutritional or health consequences will occur due to the introduction of a specific botanical preservative. Potentially sensitive populations should be recognized and included in the analysis.
An excellent discussion of factors affecting risk assessment and characterization of food supplements has been prepared by an ILSI-Europe working group (Schilter et al., 2003) and is highly recommended. In the ILSI guidance document, a decision tree is provided to assist manufacturers in determining the information that needs to be considered for the safety evaluation of a botanical ingredient or product. Those who are preparing GRAS affirmation notices should find the ILSI guidance document to be quite helpful.
A Sure Future for Biopreservatives
Botanical preservatives are only one type of a growing number of naturally occurring antimicrobial systems; others include microbial products such as bacteriocins, animal products, various enzymes, peptides, and natural sterilants. Although consumers are enamored with “naturalness,” this alone is not sufficient justification to use these new preservatives in foods.
We are currently limited to a rather small number of antimicrobial agents which have been used for many years with little expansion, and there is a real need to expand the list of preservatives which can be used to ensure safety and quality of food products. These systems lend themselves to synergistic or additive uses with one another and may also be used with conventional antimicrobial compounds and organic acids.
The future of naturally occurring antimicrobial systems seems sure, as new preservative systems are being rapidly developed and used in a variety of foods.
References
Adel, Z.M.B., Siham, M.M.F., Ahmed, T.M.E., and Barakat, S.M.M. 2002. Application of some spices in flavoring and preservation of cookies: 2. Antimicrobial and sensory properties of cardamom, cinnamon and clove. Deutsche-Lebensmittel-Rundschau 98: 261-265.
Al-Jedah, J.H., Ali, M.Z., and Robinson, R.K. 2000. The inhibitory action of spices against pathogens that might be capable of growth in a fish sauce (mehiawah) from the Middle East. Intl. J. Food Microbiol. 57: 129-133.
Argento, A., Tiraferri, E., and Marzaloni, M. 2000. Oral anticoagulants and medicinal plants. An emerging interaction. Ann. Ital. Med. Int. 15(2):139-43. Arora, D.S. and Kaur, J. 1999. Antimicrobial activity of spices. Intl. J. Antimicrob. Agents 12: 257-262.
Arora, D.S. and Kaur, J. 1999. Antimicrobial activity of spices. Intl. J. Antimicrob. Agents 12: 257-262.
Bagamboula, C.F., Uyttendaele, M., and Debevere, J. 2004. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 21: 33-42.
Ballmer-Weber, B.K., Vieths, S., Luttkopf, D., Heuschmann, P., and Wuthrich, B. 2000. Celery allergy confirmed by double-blind, placebo-controlled food challenge: A clinical study in 32 subjects with a history of adverse reactions to celery root. J. Allergy Clin. Immunol. 106: 373-378.
Bara, M.R.F. and Vanetti, M.C.D. 1995. Antimicrobial effect of spices on the growth of Yersinia enterocolitica. J. Herbs, Spices Med. Plants 3(4): 51-58.
Baydar, H., Sagdic, O., Ozkan, G., and Karadogan, T. 2004. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 15: 169-172.
Benito, M., Jorro, G., Morales, C., Pelaez, A., and Fernandez, A. 1996. Labiatae allergy: Systemic reactions due to ingestion or oregano and thyme. Annals Allergy, Asthma Immunol. 76: 416-418.
Beuchat, L.R. 1994. Antimicrobial properties of spices and their essential oils. In “Natural Antimicrobial Systems and Food Preservation.” ed. V.M. Dillion and R.G. Board. CAB Intl., Wallingford, UK.
Bisset, N.G. 1994. “Herbal Drugs and Phytopharmaceuticals, a Handbook for Practice on a Scientific Basis.” Medpharm Scientific Publishers and CRC Press, Stuttgart, Germany.
Blumenthal, M. 2001. What is herb standardization? HerbalGram 52: 25.
Blumenthal, M. 2003. Industry needs to rethink DSHEA. HerbalGram 58: 59-61.
Borris, R.P. 1996. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 51: 29-38.
Caccioni, D., Guizzardi, M., Biondi, D., Renda, A., and Ruberto, G. 1998. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Intl. J. Food Microbiol. 43: 73-79.
Cate, M. 2000. Antimicrobial and toxicological characteristics of commercial herbal extracts and the antimicrobial efficacy of herbs in marinated chicken. M.S. thesis, directed by F.A. Draughon, May, Univ. of Tennessee, Knoxville.
Cate, M. and Draughon, F.A. 2000. Antimicrobial and toxicological characteristics of commercial herbal nutraceuticals. Presented at Ann. Mtg., Inst. of Food Technologists, Dallas, Tex., June 18-21.
Cate, M., Draughon, F.A, Mount, J.R., and Golden, D.A. 2000. The antimicrobial efficacy of herbs in marinated chicken. Presented at Ann. Mtg., Intl. Assn. for Food Protection, Atlanta, Ga., Aug. 6-9.
Ceylan, E., Kang, D.E., and Fung, D.Y.C. 1998. Reduction of Escherichia coli O157:H7 in ground meat by selected spices. Presented at Ann. Mtg., Inst. of Food Technologists, Atlanta, Ga., June 20-24.
Ceylan, E., Sabah, J.R., and Fung, D.Y.C. 1999. Effect of cinnamon Escherichia coli O157:H7 in apple cider. Presented at Ann. Mtg., Inst. of Food Technologists, Chicago, Ill. July 25-28.
Chaurasi, S.C. and Vyas, K.K. 1977. In vitro effect of some volatile oil against Phytophthora parasitica var. piperina. J. Res. Indian Med. Yoga Homeopath. 1977: 24-26.
Cichewicz, R.H. and Thorpe, P.A. 1996. The antimicrobial properties of chile peppers (Capsicum species) and their use in Mayan medicine. J. Ethnolpharmacol. 52: 61-70.
Conner, D.E. 1993. Naturally occurring compounds. In “Antimicrobials in Foods,” ed. P.M. Davidson and A.L. Branen, pp. 441-450. Marcel Dekker, Inc., New York.
Conner, D.E. and Beuchat, L.R. 1984. Effects of essential oils from plants on growth of food spoilage yeasts. J. Food Sci. 49: 429-434.
Couladis, M., Chinou, I.B., Tzxakou, O., and Petrakis, P.V. 2003. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. Apollinis (Boiss. & Heldr.). Phytotherapy Res. 17(2): 152-154.
Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12: 564-582.
De, M., De, A.K., and Banerjee, A.B. 1999. Antimicrobial screening of some Indian spices. Phytotherapy Res. 13: 616-618.
Deans, S.G. and Ritchie, G. 1987. Antibacterial properties of plant essential oils. Intl. J. Food Microbiol. 5: 165-180.
Delaquis, P.J., Stanich, K., Girard, B., and Mazza, G. 2002. Antimicrobial activity of individual and mixed fractions of cilantro, coriander and eucalyptus essential oils. Intl. J. Food Microbiol. 74: 101-109.
Diaz, L., Gonzalez, C.J., Moreno, B, and Otero, A. 2002. Effect of temperature, water activity, pH and some antimicrobials on the growth of Penicillium olsonii isolated from the surface of Spanish fermented sausage. Food Microbiol. 19: 1-7.
Duke, J.A. 1985. “Handbook of Medicinal Herbs.” CRC Press, Inc., Boca Raton, Fla.
Elgayyar, M. and Draughon, F.A. 1999. Use of extracts of Nigella sativa to inhibit spoilage and pathogenic microorganisms in rainbow trout. Presented at 86th Ann. Mtg., Intl. Assn. of Milk, Food, and Environmental Sanitarians, Detroit, Mich., Aug. 1-4.
Elgayyar, M., Draughon, F.A., Golden, D.A., and Mount, J.R. 2001. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Protect. 64: 1019-1024.
FDA. 1997. Premarket notification for a new dietary ingredient. Final rule. Food and Drug Admin., Fed. Reg. 62: 49886-49892.
FDA. 2003a. Substances generally recognized as safe. Food and Drug Admin. Code of Federal Regulations, Title 21, Part 182. U.S. Govt. Printing Office, Washington, D.C.
FDA. 2003b. Summary of all GRAS notices. Center for Food Safety and Applied Nutrition, Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~rdb/opagras.html, accessed under FDA on 1/12/04.
FDA. 2003c. Food labeling: Ingredient labeling of dietary supplements that control botanicals. Proposed rule. Food and Drug Admin., Fed. Reg. 68: 51738-51749.
FDA. 2003d. Food labeling. Food and Drug Admin. Code of Federal Regulations, Title 21, Part 101.22. U.S. Govt. Printing Office, Washington, D.C.
FDA. 2003e. Current good manufacturing practice in manufacturing, packing or holding dietary ingredients and dietary supplements. Proposed rule. Food and Drug Admin., Fed. Reg. 68: 12157-12263.
Gill, A.O., Delaquis, P., Russo, P., and Holley, R.A. 2002. Evaluation of antilisterial action of cilantro oil on vacuum packaged ham. Intl. J. Food Microbiol. 73: 83-92.
Greuter, W., McNeill, J., Barrie, F.R., Burdet, H.M., Demoulin, V., Filgueiras, T.S., Nicolson, D.H., Silva, P.C., Skog, J.E., Trehane, P., Turland, N.J., and Hawksworth, D.L. 2000. “International Code of Botanical Nomenclature (St. Louis Code).” Koeltz Scientific Books, Konigstein, Germany.
Hall, T., Lu, Z., Yat, P., Fitzloff, J., Arnason, J., Awang, D., Fong, H., and Blumenthal, M. 2001. Evaluation of consistency of standardized Asian ginseng products in the ginseng evaluation program. HerbalGram 52: 31-46.
Hancock, R.T. and Harrison, M.A. 2000. Antimicrobial activity of selected spices and organic acids against Arcobacter butzleri in laboratory media and on fresh pork. www.griffin.peachnet.edu/cfs/Pages/Research/PorkResearch.html, accessed 7/10/2003.
Hata, M., Sasaki, E., Ota, M., Fujimoto, K., Yajima, J., Shichida, T., and Honda, M. 1997. Allergic contact dermatitis from turmeric. Allergic contact dermatitis from curcumin (turmeric). Contact Dermatitis 36:107-108.
Heck, A.M., DeWitt, B.A., and Lukes, A.L. 2000. Potential interactions between alternative therapies and warfarin. Am. J. Health Syst. Pharm. 57:1221-1227.
Hoult, J.R.S. and Paya, M. 1996. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 27: 713-722.
Hughes, K. 2000. Botanicals—Caught between two worlds. Prepared Foods 169: 13-18.
Huhtanen, C.N. 1980. Inhibition of Clostridium botulinum by spice extracts and aliphatic alcohols. J. Food Protect. 43: 195-196.
Kanerva, L. 2001. Skin contact reactions to spices. A review. Acta Dermatovenerolgica 10: 1-7. www.mf.uni-lf.si/acta-apa/acta-apa-01-1/1-clanek.html, accessed 1/15/2004.
Kanerva, L., Estlander, T., and Jolanki, R. 1996. Occupational allergic contact dermatitis from spices. Contact Dermatitis 35:157-162.
Kizil, S. and Sogut, T. 2002. An investigation on the antimicrobial effects of essential oils of coriander and cumin at different concentrations. Turkish J. Field Crops 7: 1-5.
Lambert, R.J.W., Skandamis, P.N., Coote, P.J., and Nychas, G.J.E. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91: 453-562.
Lemay, M.J., Choquette, J., Delaquis, P.J., Gariepy, C., Rodrigue, N., and Saucier, L. 2002. Antimicrobial effect of natural preservatives in a cooked acidified chicken meat model. Intl. J. Food Microbiol. 78: 217-226.
Leuschner, R.G.K. and Zamparini, J. 2002. Effects of spices on growth and survival of Escherichia coli O157 and Salmonella enterica serovar Enteritidis in broth model systems and mayonnaise. Food Control 13: 399-404.
Lewis, C.J. 2001. Letter to manufacturers regarding botanicals and other novel ingredients in conventional foods, Jan. 30. Center for Food Safety and Nutrition, Office of Nutritional Products, Labeling and Dietary Supplements, Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~dms/ds-ltr15.html, accessed 1/9/2004.
Manderfeld, M.M., Schafer, H.W., Davidson, P.M., and Zottola, E.A. 1997. Isolation and identification of antimicrobial furocoumarins from parsley. J. Food Protect. 60: 72-77.
McGuffin, M., Kartesz, J., Leung, A., and Tucker, A. 2000. “Herbs of Commerce,” 2nd ed. Am. Botanical Council, Austin, Tex.
Meena, M.R. and Sethi, V. 1994. Antimicrobial activity of essential oils from spices. J. Food Sci. Technol. 31: 68-70.
Nielsen, P.V. and Rios, R. 2000. Inhibition of fungal growth on bread by volatile components from spices and herbs and the possible application in active packaging, with special emphasis on mustard essential oil. Intl. J. Food Microbiol. 60: 219-229.
Okouchi, S., Fukusima, Y., and Sugita, H. 2002. Calorimetric evaluation of antimicrobial activities for essential oils. Aroma Res. 3(2): 117-121.
Ozkan, G., Sagdic, O., and Ozcan, M. 2003. Note: Inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci. Technol. Intl. 9(2): 85-88.
Peres, M.T., Monache, F.D., Cruz, A.B., Pizzolatti, M.G., and Yunes, R.A. 1997. Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 56: 223-226.
Ponce, A.G., Fritz, R., del Valle, C., and Roura, S.I. 2003. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Lebensm-Wiss-u.-Technol. 36: 679-684.
Prasad, M.M. and Seenayya, G. 2000. Effect of spices on growth of red halophilic cocci isolated from salt cured fish and solar salt. Food Res. Intl. 33: 793-798.
Pszczola, D.E. 2001. 2001: A spice odyssey. Food Technol. 55(1): 36-44.
Pszczola, D.E. 2002. Beefing up innovations for meat and poultry ingredients. Food Technol. 56(3): 54-67.
Rahman, A. and Choudhary, M.I. 1995. Diterpenoid and steroidal alkaloids. Nat. Prod. Rep. 12: 361-379.
Rath, C.C., Dash, S.K., and Mishra, R.K. 2002. Antibacterial efficacy of six Indian essential oils individually and in combination. J. Essential Oil Bearing Plants 5(2): 99-107.
Sagdic, O. and Ozcan, M. 2003. Antibacterial activity of Turkish spice hydrosols. Food Control 14(3): 141-143.
Sangalli, B.C. and Chiang, W. 2000. Nutmeg contains several compounds with structural similarities to substances with known central nervous system neuromodulatory activity. J. Toxicol. Clin. Toxicol. 38: 671-678.
Scheiper, R. 1993. Hot spice. Haarman & Reimer, Springfield, N.J.
Schilter, B., Andersson, C., Anton, R., Constable, A., Kleiner, J., O’Brien, J., Renwick, A.G., Korver, O., Smit, F., and Walker, R. 2003. Guidelines for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem. Toxicol. 41: 1625-1649.
Schultes, R.E. 1978. The kingdom of plants. In “Medicines from the Earth,” ed. W.A.R. Thompson, p. 208. McGraw-Hill Book Co., New York.
Schwartz, H.J., Jones, R.T., Rojas, A.R., Squillace, D.L., and Yunginger, J.W. 1997. Occupational allergic rhinoconjunctivitis and asthma due to fennel seed. Ann. Allergy Asthma Immunol. 78(1): 37-40.
Servan, J. 1998. Hallucinations after voluntary ingestion of nutmeg: An unrecognized drug abuse. Rev. Neurol. (Paris). 154(10): 708.
Suhr, K. and Nielsen, P.V. 2003. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 94: 665-674
Takikawa, A., Abe, K., Yamamoto, M., Ishimaru, S., Yasui, M., Okubo, Y., and Yokoigawa, K. 2002. Antimicrobial activity of nutmeg against Escherichia coli O157. J. Biosci. Bioeng. 94: 315-320.
Tepe, B., Donmez, R., Unlu, M., Candan, F., Daferera, D., Vardar-Unlu, G., Polissiou, M., and Sokmen, A. 2004. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Monbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 84: 519-525.
Toda, M., Okubo, S., Ikigai, H., Suzuki, T., Suzuki, Y., Hara, Y, and Shimamura, T. 1992. The protective activity of tea catechins against experimental infection by Vibrio cholerae 01. Microbiol. Immunol. 36: 999-1001.
Tsigarida, E., Skandamis, P., and Nychas, G-J.E. 2000. Behavior of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. J. Appl. Microbiol. 89: 901-909.
Verkerk, R. 2003. The European Union Directives—How they impact innovation in nutritional medicine. J. Nutr. Env. Med. 13(2): 75-77.
Zaynoun S, Abi Ali, L, Tenekjian, K, and Kurban A. 1985. The bergapten content of garden parsley and its significance in causing cutaneous photosensitization. Clin. Exp. Dermatol. 10: 328-331.
