GRAS Flavoring Substances 22
The 22nd publication by the FEMA Expert Panel presents safety and usage data on 185 new generally recognized as safe flavoring ingredients and describes an approach to assessing the safety of natural flavor complexes.
The Flavor and Extract Manufacturers Association’s GRAS Program is now in its 45th year of continuous operation. During that time, the FEMA Expert Panel, as an independent scientific body, has rigorously evaluated the safety of food flavors with the goal of protecting human health.

The GRAS program was established to respond to the provision in the 1958 Food Additives Amendment to the Federal Food, Drug, and Cosmetics Act—Public Law 85-929, 72 Stat. 1784 (1958), codified at 21 USC Section 348 (1988)—that exempted from food additive status those substances “generally recognized by experts qualified by scientific training and experience to evaluate its safety, as having been adequately shown through scientific procedures . . . to be safe under the conditions of intended use.” Based on this provision, substances “generally recognized as safe” (GRAS) by the FEMA Expert Panel are not considered to be food additives, and are excluded from mandatory premarket approval by the Food and Drug Administration (Hallagan and Hall, 1995).
For most of the Program’s history, the Expert Panel has concentrated on the evaluation and regularly scheduled reevaluation of data related to the safe use of approximately 1,900 chemically identified flavor ingredients. However, over the past decade, the Panel recognized that a comprehensive GRAS program for food flavorings entails not only the consideration of chemically identified flavoring substances, but also the assessment of natural flavor complexes such as essential oils, extracts, and oleoresins.
The Panel also recognized that other chemical substances with non-flavor function—such as emulsifiers, antioxidants, and flavor modifiers—are used in the preparation of finished flavors. Most finished flavors are a combination of chemically identified substances, natural flavor complexes, and those substances (stabilizers, solvents, and emulsifiers) required to process the flavor into the finished food product. Since finished flavorings function optimally at such low levels in foods, intake of these three types of substances normally represents a minute contribution to the diet compared to direct food additives and food itself.
As one of its principal long-term goals, the Panel strives to develop scientifically rigorous criteria and procedures that can be used to evaluate the safety of all substances used in the production of food flavors. Over the past four decades, criteria have been developed to evaluate the safety of nearly 1,900 substances (Woods and Doull, 1991; Smith et al., 2005b). The scientific criteria used by the Panel to reach its GRAS conclusions were last addressed in 1991 (Woods and Doull, 1991). Scientific advances since 1991 in the areas of DNA adduct formation, experimental pathology, and molecular mechanism of genotoxicity and mixture safety evaluation now play a major role in understanding the chemical, biochemical, and biological fate of flavoring substances consumed in food.
Given the impact of these recent scientific advances, primarily in fields related to molecular reactions in vivo, revised guidelines for current and future GRAS decisions have recently been published (Smith et al., 2005b). These criteria are vigorously applied to evaluate chemically identified substances, natural flavor complexes, and substances exhibiting non-flavor function used in the preparation of finished food flavors.
Because of the rich database of safety data available, the evaluation of substances needed to stabilize, emulsify, acidify, etc., compounded flavorings followed more-traditional lines of safety evaluation. Almost without exception, these substances possess extensive toxicity data. These data provide enormous margins of safety for the intake of low levels of these substances from use in flavors. These margins of safety are orders of magnitude lower than those for the same substances added directly to food.
The most difficult aspect of safety evaluation for flavors involves naturally occurring mixtures or natural flavor complexes. The development of scientifically based criteria to evaluate naturally occurring mixtures such as essential oils provides a significant challenge, one that mixture toxicology has faced for decades. The recent publication of a guide to evaluate natural flavor complexes (NFCs), specifically essential oils (Smith et al., 2005a), is a major step forward. The practical procedure is based mainly on the evaluation of the chemical composition of the NFC and the variability of that composition in the commercially available product. It represents the first attempt to evaluate the safety of a naturally occurring complex chemical mixture based on its actual chemical composition and safety data available for those constituents.
--- PAGE BREAK ---
The GRAS Reaffirmation Program
In 1994, the Panel initiated its third comprehensive review of data relevant to the safety of all chemically identified GRAS flavoring substances. As in the previous comprehensive review (GRAS affirmed or GRASa) performed between 1979 and 1988, the Panel evaluated each flavoring substance within the context of safety data on the group of structurally related substances. This group approach involves the evaluation of data available for the specific substance and a much larger volume of data for structurally related substances that are anticipated to exhibit similar chemical, biochemical, and biological fate, especially at low levels of intake from their intended use as flavors.
Using newly revised evaluation criteria (Smith et al., 2005a), the Panel reaffirmed the GRAS status of more than 98% of chemically identified GRAS substances. In relatively few cases, the Panel requested that additional safety studies be performed for representative substances (e.g., verbenone and nootkatone) in a selected chemical group (bridged and fused bicyclic ketones) prior to successful completion of the GRASr evaluation of that chemical group.
The GRASr program is now essentially complete, and the results are presented in this article. Through 2004, more than 1,900 chemically identified flavoring substances have been reevaluated and recognized as GRASr.
As part of the GRASr program, the key scientific data on which the GRASr decisions are based is published in the peer-reviewed literature. These published data have been organized according to chemical group. For example, the safety data for chemical groups such as aliphatic and aromatic lactones, cinnamyl, phenethyl alcohol, benzyl, and hydroxybenzyl derivatives have been published (Adams et al., 1996, 1997, 1998, 2004; Newberne et al., 1999; Smith et al., 2002a, b). These publications contain an analysis of food and flavor exposure data; data on absorption, distribution, metabolism, excretion, and molecular reactions with proteins and DNA; and reports of toxicology, carcinogenicity, and genotoxicity studies for groups of structurally related flavoring substances. These are, in addition >>> to expert judgment, the key data on which GRAS and GRASr decisions are made.
The Most New Substances Since 1965
In addition to the reevaluation of safety data on existing GRAS substances, a significant number of new candidates have been evaluated as GRAS for their intended use as flavoring substances. The main reason for the recent influx of GRAS candidates is related to international interest in developing a global positive list of flavoring substances. Flavor safety evaluation programs begun by the World Health Organization/Food Agriculture Organization (WHO/FAO) Joint Expert Committee on Food Additives (JECFA) and in Europe are based on scientific principles similar to those used by the FEMA Expert Panel over the past four decades. Therefore, in the spirit of globalization, FEMA recommended that the current GRAS system that had previously been limited to United States FEMA-member companies should be expanded to allow foreign producers of flavors to submit flavoring substances for GRAS evaluation and eventual sale in the U.S. marketplace.
As a result, FEMA created the International Group GRAS Program. In this program, flavor companies that are members of the International Organization of Flavor Industries (IOFI) or their national flavor trade associations can submit groups of structurally related flavoring substances for GRAS evaluation by the FEMA Expert Panel. Since its inception in 2002, the Group GRAS Program is the major reason that GRAS 22 contains the most new chemically identified flavoring substances (185) since the first GRAS list (GRAS 3) was published in 1965.
For the vast majority of substances in the GRASr Program, the conclusions concerning the GRAS status of chemically identified flavoring substances have been reaffirmed by another evaluating body. Beginning in 1996, JECFA began a program to evaluate chemically identified flavoring substances. Through 2004, it has evaluated approximately 1,500 flavoring substances.
JECFA has annually evaluated groups of structurally related chemically identified flavoring substances (130–220/year) using a procedure presented at JECFA in 1995 and formally adopted in 1996 (JECFA, 1997). Initial evaluations during 1996 were time consuming, in that each substance was evaluated individually. However, JECFA determined that efficient but effective evaluations could be performed on groups of chemically related substances similar to but not identical to those used by the Expert Panel. Based on this group approach and use of a formal evaluation procedure, JECFA has reached the conclusion that the reviewed flavoring substances are safe under current conditions of intake.
--- PAGE BREAK ---
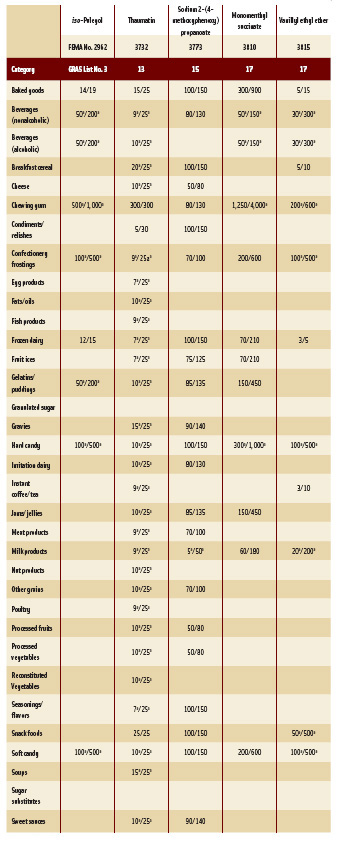 In this, the 22nd GRAS publication, 185 new GRAS flavoring substances are identified—FEMA Nos. 4069–4253 (Tables 1 and 2, pp. 40–60). In addition, the Panel determined that new use levels and food categories for five flavoring substances are consistent with their current GRAS status (Table 3). Of these 185 new flavoring substances, four (Nos. 4219–4222) are NFCs, and six (Nos. 4223–4229) are substances with a non-flavor function that are used in the preparation of finished food flavors.
In this, the 22nd GRAS publication, 185 new GRAS flavoring substances are identified—FEMA Nos. 4069–4253 (Tables 1 and 2, pp. 40–60). In addition, the Panel determined that new use levels and food categories for five flavoring substances are consistent with their current GRAS status (Table 3). Of these 185 new flavoring substances, four (Nos. 4219–4222) are NFCs, and six (Nos. 4223–4229) are substances with a non-flavor function that are used in the preparation of finished food flavors.
GRAS 22 also presents elements of the guide for the safety evaluation of NFCs composed exclusively of volatile constituents (essential oils and distillates) and its application to the GRAS evaluation of corn mint oil, Mentha arvensis L. and the GRASr evaluation of lemongrass oil, Cymbopogon citratus (DC) Stapf. and Cymbopogon flexuosus (Nees ex. Steud.).
Safety Assessment of Natural Flavor Complexes
Publication of GRAS 18 (Newberneet al., 1998) provided the first insights into the Panel’s approach to the safety evaluation of NFCs. Fundamentally, biological responses are the result of the in-vivo interactions of one or more molecules in an NFC or their metabolites with macromolecules (proteins, enzymes, etc.). Molecules exert their flavor function by binding to receptor proteins of the gustatory or olfactory systems at extremely low levels of exposure. It has been estimated that as few as 40 molecules produces an identifiable sensation (Devries and Stuiver, 1961). When distributed in the body at much higher levels, these same molecules—and, in some cases, their metabolites—bind an assortment of proteins and other macromolecules, potentially leading to toxicity.
Based on years of experience reviewing the database of information on NFCs and their chemically identified constituents, the Panel has concluded that a scientifically based evaluation of an NFC should involve a comprehensive evaluation of available data for the NFC and the chemical constituents that make up the NFC. The method for a constituent-based safety evaluation of essential oils is the subject of recent publications (Smith et al., 2004, 2005a).
Previous safety evaluations of NFCs have followed more-traditional approaches. Typically, a representative sample of the NFC with recognized physical specifications was subjected to a battery of toxicology tests. If sufficient margins of safety existed between estimated intakes of the NFC and no-effect levels in animal studies, intake of the NFC was concluded to be safe. Of course, key assumptions are inherent in the standard toxicologic approach: (1) the sample of an NFC to be tested is representative of the product in the marketplace now or at some point in the future; (2) NFCs are derived from nature and subjected to further physical processing (i.e., distillation, blending, etc.); and (3) there is without exception variability in the composition that is not accounted for in the testing of a single sample. Treating an NFC as a single chemical entity leaves no flexibility for evaluating the safety of the full range of product used in the marketplace over an extended period of time.
For certain NFCs, a comprehensive chemical characterization may not always be practical or even possible. In these instances, the standard toxicological approach may be the only viable option for determining safety in use. However, given the advances in low-cost, high-throughput technology to identify and quantify NFC constituents, it is more often feasible to chemically characterize a full range of commercial samples of an essential oil over an extended period of time than to subject the mixture to a standard battery of toxicity tests for a product that may not e fully representative of the product in commerce now or in the future.
The guide is a chemically based procedure for the safety evaluation of existing GRAS NFCs and new candidate GRAS NFCs for their intended use as flavoring substances (Smith et al, 2005b). In its current form, the guide is limited to essential oils and distillates in which essentially all of the mass of the NFC is composed of volatile constituents. The procedure will almost certainly undergo revisions and refinements, given its early stage of development and, more importantly, this endeavor is the first practical but exhaustive attempt to evaluate naturally occurring complex mixtures based on their actual chemical composition and the variability of that composition for the product in commerce.
The guide considers the safety of all chemically identified and unidentified chemical constituents of the essential oil with the intent that no significant part of the oil goes unevaluated. Since experience in toxicology, biochemistry, chemistry, and pathology and a thorough knowledge of natural products chemistry and structure–activity relationships all play a major role in the evaluation, a broad range of scientific expertise and judgment are required to successfully apply this guide to essential oils.
--- PAGE BREAK ---
Extensive data on botanical origin, physical properties, isolation processes, intake of the essential oil, and qualitative and quantitative analytical data for the chemical composition of the product in commerce are required for the successful use of the guide. To effectively evaluate an essential oil, attempted complete analyses (analyses for all of the constituents) must be available for the product intended for the marketplace. The industry is therefore obligated to collect analytical data on many different samples of a commercial essential oil to ensure that the evaluation is representative of the flavoring product used worldwide.
Additional quality control data of key flavor constituents is useful, since it demonstrates consistency in the chemical composition of the product being marketed over time. These composition data are not to be confused with analytical data collected for the crude oil isolated in the field. These analytical data are used mainly to improve field crop yields and oil yields. Only those data collected on the finished essential oil intended for their addition as flavorings to food are used as the basis for the safety evaluation.
Steps in Safety Evaluation
Smith et al. (2005a) provides a detailed discussion of each step of the guide. The discussion below is an outline of the guide and a summary of the results of the evaluation for a new GRAS essential oil, corn mint oil, and the GRAS reaffirmation of lemongrass oil. Basically, the guide consists of five parts:
1. Comparison of the intake of an essential oil (e.g., basil oil) intentionally added to food and the intake of the oil as the result of consuming the plant source as a food (e.g., basil). Presumably, if the intake of the oil occurs principally through consumption as a food and not from added flavor use, then the concern for safety of the flavor use of the oil is significantly reduced.
However, in cases such as corn mint oil, in which the plant is not consumed primarily as a food, a more rigorous set of criteria are applied to the evaluation, especially the unidentified constituents. This step is followed by a series of steps that organize and prioritize the constituents for further evaluation.
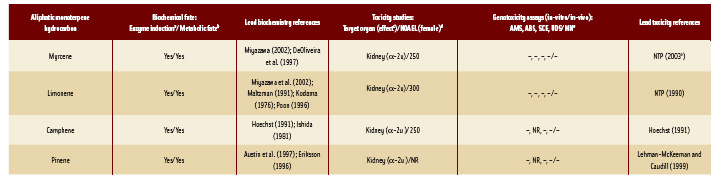 2. The chemically identified constituents are assigned to congeneric groups, and each group is prioritized according to its relative intake from consumption of the oil as a flavor, its metabolic fate, and its potential toxicity (Structural Classes I, II, or III; Cramer et al., 1978; Munro et al., 1996). For more than a decade, the Panel has used toxicity and metabolic data to organize the large number of chemically identified GRAS substances (>1,900), the majority of which are naturally occurring, into congeneric groups (e.g., aliphatic terpene hydrocarbons; limonene, campene, myrcene, pinene, etc.) that exhibit similar metabolic fate and toxic potential. Biochemical data support the conclusion that substances in the group undergo enzyme-catalyzed reactions to yield metabolites that are readily excreted or further metabolized to carbon dioxide and water. Toxicity data support the conclusion that the differences in toxic potency between members of a congeneric group are small compared to the large differences between intake of members of the group as flavorings and toxic dose levels. Additionally, consistent toxicity and genotoxicity data demonstrate that small molecular changes among members of a congeneric group generally do not significantly alter toxic potency (Table 4).
2. The chemically identified constituents are assigned to congeneric groups, and each group is prioritized according to its relative intake from consumption of the oil as a flavor, its metabolic fate, and its potential toxicity (Structural Classes I, II, or III; Cramer et al., 1978; Munro et al., 1996). For more than a decade, the Panel has used toxicity and metabolic data to organize the large number of chemically identified GRAS substances (>1,900), the majority of which are naturally occurring, into congeneric groups (e.g., aliphatic terpene hydrocarbons; limonene, campene, myrcene, pinene, etc.) that exhibit similar metabolic fate and toxic potential. Biochemical data support the conclusion that substances in the group undergo enzyme-catalyzed reactions to yield metabolites that are readily excreted or further metabolized to carbon dioxide and water. Toxicity data support the conclusion that the differences in toxic potency between members of a congeneric group are small compared to the large differences between intake of members of the group as flavorings and toxic dose levels. Additionally, consistent toxicity and genotoxicity data demonstrate that small molecular changes among members of a congeneric group generally do not significantly alter toxic potency (Table 4).
3. In the next part, the intake of each congeneric group is evaluated with respect to metabolic pathways available for the safe disposition of the members of the group. If an intoxication pathway has been identified that may play a significant role in the safe disposition of the substance, this is cause to separate that substance or substances into a different congeneric group (see pulegone, below).
--- PAGE BREAK ---
The congeneric group is assigned to a structural class (Cramer et al., 1978) that relates structure to potential toxicity. If the group (e.g., aliphatic monoterpene hydrocarbons) has structural features that allow for its efficient and rapid detoxication, it is assigned to Structural Class I. If its structural features or those of a principal metabolite indicate intoxication (e.g., 2-isopropylidenecyclohexanones, pulegone), it is assigned to Structural Class III. Chemicals of questionable biochemical and biological fate are assigned to Structural Class II.
Based on these three structural classes, the intake of the congeneric group is then compared to thresholds for toxicity (human exposure thresholds) obtained from a large database of no-observable-effect levels (NOELs) for a wide range of chemical substances (Munro et al., 1996). This step involves a comparison of intake for the congeneric group to no-effect levels for a large group of substances in the same structural class. If the intake exceeds 100 times the 5th-percentile NOEL (i.e., only 5% of the substances in the structural class have lower NOELs), toxicity data for representative members of the group are required.
The NOELs for these substances (e.g., menthol and menthone) are compared to the intake of the group (alicyclic secondary alcohols/ketones and related esters) to ensure that adequate margins of safety exist between intake of the group and no-effect levels in toxicity studies. At no point does the evaluation involve isolated consideration of data on an individual constituent. Rather, congeneric groups are evaluated in the context of all data available for members of the group.
In terms of effort, instead of individually evaluating each of the chemically identified constituents in isolation (e.g., 150 constituents of corn mint oil), the guide focuses on the comprehensive evaluation of a few congeneric groups (3–8) containing these constituent chemicals.
4. Subsequently, the total intake of unidentified constituents is evaluated by comparing intake through added flavor use to intake from consumption of food. If intake through food use exceeds added flavor use, there is little concern for the intake of the unidentified constituents. However, if intake of the essential oil is not principally from intake of food, the intake is compared to the most conservative exposure threshold (90 μg/person/day for Structural Class III).
If intake is toxicologically insignificant (<90 μg/person/day), then safety concern for intake of unidentified constituents is reduced. If intake of the unidentified constituents exceeds this level, then toxicity data on the essential oil or an oil of similar composition is required, or additional analyses must be obtained to reduce the number and intake of unidentified constituents in the essential oil.
Although the unidentified constituents are placed in the highest class of toxicological concern, it does not necessarily mean that the structures of these substances present a safety concern. In reality, many of the unidentified constituents are expected to belong to the same congeneric groups in the NFC. They often represent shoulders on a gas or liquid chromatographic peak for a constituent of known structure, possibly a double-bond isomer or dihydro derivative. It is noteworthy that most newly identified naturally occurring constituents (Nijssen et al., 2003) are structurally related to constituents previously identified in that NFC. Therefore, the assignment of these substances to Structural Class III is a conservative default feature of the guide.
5. When the evaluation of the intake of each congeneric group and the total intake of unidentified constituents is completed, the essential oil itself is evaluated in the context of the combined intake of all congeneric groups and the total of unidentified constituents, plus any other related data (e.g., data on the essential oil itself or on an essential oil of similar composition). The guide organizes the extensive database of information on the NFC to efficiently evaluate the essential oil under conditions of intended use. It is, however, not intended to be a rigid checklist. The Panel will continue to evaluate each essential oil on a case-by-case basis, applying their scientific judgment to ensure that no significant part of the NFC goes unevaluated.
An important issue for the flavor industry is the relationship of the results of the safety evaluation to the specifications for the essential oil placed into commerce. An essential oil produced under good manufacturing practices (GMPs) should be of a purity (quality) and chemical composition sufficiently high to represent a reasonable certainty of safety under conditions of intended use. In addition to specifying the biological origin, physical and chemical properties, and other identifying characteristics of the essential oil, specifications must include the chemical assay for the essential oil in commerce to link the chemical composition of the essential oil to the safety evaluation.
--- PAGE BREAK ---
The chemical assay requirements should not create an undue burden on the industry and should be easily incorporated into an ongoing quality control program that monitors key constituents reflecting flavor function of an essential oil. They should be consistent with other published specifications for chemical assay such as those listed for essential oils in the 5th edition of Food Chemicals Codex (FCC, 2003) and those of the International Organization for Standardization (ISO).
To meet the above conditions and be consistent with the results of the safety evaluation, the chemical assay should specify (1) upper limits of concentrations for key congeneric groups that constitute the vast majority of the oil; (2) key constituents in congeneric groups that would be monitored in an ongoing quality control program that also reflect the technical flavor function of the product (e.g., linalool in coriander oil or menthol in peppermint oil); and (3) information on constituents exhibiting a higher order of toxicity or the group of unidentified constituents that may be of a safety concern at sufficiently high levels of intake (e.g., pulegone in peppermint oil, Mentha piperita).
The scope of a specification should be sufficient to ensure safety in use, but not impose an obligation on the industry to perform ongoing analyses for constituents unrelated to the safety or to the flavor function of the essential oil.
 This approach is illustrated below by the safety assessment of corn mint oil and lemongrass oil. The daily per capita intake is calculated by the equation shown in the accompanying graphic.
This approach is illustrated below by the safety assessment of corn mint oil and lemongrass oil. The daily per capita intake is calculated by the equation shown in the accompanying graphic.
Safety Assessment of Corn Mint Oil
Corn mint oil (FEMA No. 4219) is produced by the steam distillation of the flowering herb of M. arvensis. The crude oil contains more than 70% (–)-menthol, some of which is isolated by crystallization at low temperature. The resulting dementholized oil is corn mint oil.
Although produced mainly in Brazil during the 1970s and 1980s, corn mint oil is now produced predominantly in China and India. Corn mint oil has a more stringent taste compared to that of peppermint oil, M. piperita, and therefore is a cheaper substitute for peppermint oil.
Corn mint oil isolated from various crops undergoes subsequent “clean up,” further distillation, and blending to produce the finished commercial oil. The analytical data cited below are representative of samples of commercial oil intended for use in flavorings. Although there may be significant variability in the concentrations of individual constituents in different samples of crude essential oil, there is far less variability in the concentration of constituents and congeneric groups in the finished commercial oil. Also, corn mint oil is not normally consumed as a food. Therefore, the total of unidentified constituents must be considered in light of data on intake, toxicity thresholds, and actual toxicity data for the oil or an oil of similar chemical composition.
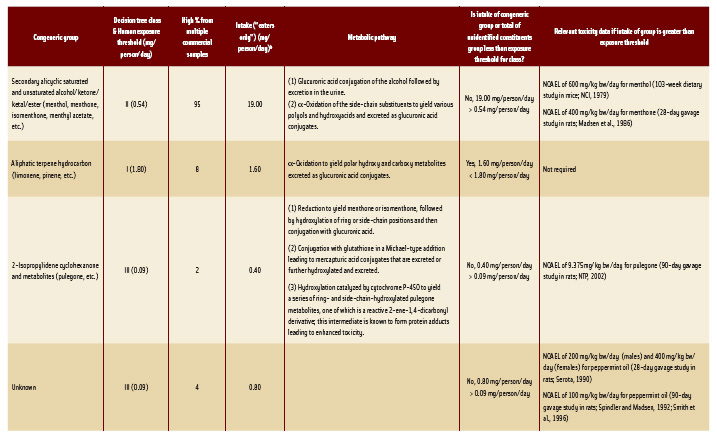 • Principal Congeneric Group. In corn mint oil, the principal congeneric group is composed of terpene alicyclic secondary alcohols, ketones, and related esters, as represented by the presence of (–)-menthol, (–)-menthone, (+)-isomenthone, (–)-menthyl acetate, etc. Samples of triple-distilled commercial corn mint oil may contain up to 95% of this congeneric group. The biochemical and biological fate of this group of substances has been previously reviewed (Adams et al., 1996; JECFA, 1999). Key data on metabolism, toxicity, and carcinogenicity are cited in Table 5 to complete the evaluation.
• Principal Congeneric Group. In corn mint oil, the principal congeneric group is composed of terpene alicyclic secondary alcohols, ketones, and related esters, as represented by the presence of (–)-menthol, (–)-menthone, (+)-isomenthone, (–)-menthyl acetate, etc. Samples of triple-distilled commercial corn mint oil may contain up to 95% of this congeneric group. The biochemical and biological fate of this group of substances has been previously reviewed (Adams et al., 1996; JECFA, 1999). Key data on metabolism, toxicity, and carcinogenicity are cited in Table 5 to complete the evaluation.
Corn mint oil is anticipated to develop a market of 200,000 kg/year, or approximately 10% of the market for peppermint oil, which corresponds to a daily per capita intake (“eaters only”) of approximately 20 mg/person/day (0.333 mg/kg bw/day) of corn mint oil. Although constituents in this group are effectively detoxicated via conjugation of the corresponding alcohol or ω-oxidation followed by conjugation and excretion (Yamaguchi et al., 1994; Madyastha and Srivatsan, 1988; Williams, 1940), the intake of the congeneric group (19 mg/person/day) is higher than the exposure threshold of 0.54 mg/person/day for Structural Class II. Therefore, toxicity data are required for this congeneric group.
--- PAGE BREAK ---
In both long- and short-term studies (Madsen et al., 1986; NCI, 1979), menthol, menthone, and other members of the group exhibit no-observable-adverse-effect levels (NOAELs) at least 1,000 times the daily per capita intake (“eaters only”) (0.32 mg/kg bw/day) of this congeneric group resulting from intake of the essential oil. Numerous in-vitro and in-vivo genotoxicity assays are consistently negative (Heck et al., 1989; Sasaki et al., 1989; Muller, 1993; Florin et al., 1980; Rivedal et al., 2000, Zamith et al., 1993; NTP, 2003a) for members of this group. Therefore, the intake of this congeneric group from consumption of M. arvensis is not of a safety concern.
• Pulegone. Although it is a constituent of corn mint oil and is also a terpene alicyclic ketone structurally related to the above congeneric group, pulegone exhibits a unique structure (i.e., 2-isopropylidenecyclohexanone) that participates in a well-recognized intoxication pathway (McClanahan et al., 1989; Thomassen et al., 1992; Adams et al., 1996; Chen et al., 2001) leading to hepatotoxicity at intake levels at least an order of magnitude less than the NOELs for structurally related alicyclic ketones and secondary alcohols (menthone, carvone, and menthol). Therefore, pulegone and its metabolite (menthofuran) that account for <2% of commercial corn mint oil are considered separately. In this case, the daily per capita intake (“eaters only”) of 0.40 mg/person/day (0.0067 mg/kg bw/day) exceeds the 0.09 mg/kg bw/day threshold for Class III. However, a 90-day study on pulegone (NTP, 2002) showed a NOAEL (9.375 mg/kg bw/day) that is approximately 1,000 times the intake of pulegone and its metabolites as constituents of corn mint oil.
Also, in a 28-day study (Serota, 1990) with peppermint oil, M. piperita, containing approximately 4% pulegone and menthofuran, a NOAEL of 200 mg/kg bw/day for male rats and a NOAEL of 400 mg/kg bw/day for female rats were established, which corresponds to a NOAEL of 8 mg/kg bw/day for pulegone and menthofuran. In a 90-day study with a mixture of M. piperita and M. arvenesis peppermint oils (Splindler and Madsen, 1992; Smith et al., 1996a), a NOAEL of 100 mg/kg bw/day was established, which corresponds to a NOAEL of 4 mg/kg bw/day for pulegone and menthofuran.
• Terpene Hydrocarbons. The only other congeneric group that accounts for >2% of the composition of corn mint oil is a congeneric group of terpene hydrocarbons: (+) and (–)- pinene, (+) limonene, etc.). Although these may contribute up to 8% of the oil, upon multiple redistillations during processing the hydrocarbon content can be significantly reduced (<3%) in the finished commercial oil. Using the 8% figure to determine a conservative estimate of intake, the intake of terpene hydrocarbons is 1.6 mg/person/day (0.027 mg/kg bw/day).
This group is predominantly metabolized by CYP P-450–induced hydroxylation and excretion in conjugated form (Ishida et al., 1981; Madyastha and Srivatsan, 1987; Crowell et al., 1994; Poon et al., 1996; Vigushin et al., 1998; Miyazawa et al., 2002). The daily per capita intake (1.60 mg/person/day) is slightly less than the exposure threshold (1.80 mg/person/day) for Structural Class I. Although no additional data would be required to complete the evaluation of this group, NOAELs (300 mg/kg bw/day) from long-term studies (NTP, 1990) on principal members of this group are orders of magnitude greater than the daily per capita intake (“eaters only”) of terpene hydrocarbons (0.025 mg/kg bw/day). Therefore, all congeneric groups in corn mint oil are considered safe for use when consumed in corn mint oil.
• Unidentified Constituents. The total of unidentified constituents in commercial corn mint oil range from a low of 2.9% up to 4%. This corresponds to a daily per capita intake (“eaters only”) of up to approximately 0.80 mg/person/day). This exceeds the 0.09 mg/person/day for the structural class of highest toxic concern (Class III). Therefore toxicity data are required on the essential oil or one of a similar chemical composition. A 28-day study on peppermint oil containing essentially the same constituents as corn mint oil shows NOAELs of 100 mg/kg/day for the oil, equivalent to 5 mg/kg bw/day for the unidentified constituents (Serota, 1990), which is at least 100 times the intake (0.0133 mg/kg bw/day) of unidentified constituents present in corn mint oil. Hence the total of unidentified constituents is also not a safety concern.
• The Essential Oil. Finally, the essential oil itself is evaluated in the context of the combined intake of all congeneric groups and the total of unidentified constituents, and any other related data. Interestingly, members of the terpene alicylic secondary alcohols, ketones, and related esters, multiple members of the monoterpene hydrocarbons, and peppermint oil itself show a common nephrotoxic effect recognized as α-2u-globulin nephropathy. The pathologists on the Panel evaluated kidney data for male rats in the mint oil study and determined that the data were consistent with the presence of α-2u-globulin nephropathy.
--- PAGE BREAK ---
In addition, a standard immunoassay for detecting the presence of α-2uglobulin was performed on kidney sections from male and female rats in the mint oil study (Serota, 1990). Results of the assay confirmed the presence of α-2u-globulin nephropathy in male rats (Swenberg and Schoonhoven, 2002). This effect is found only in male rats and is not relevant to the human health assessment of corn mint oil. Other toxic interactions between congeneric groups are expected to be minimal, given that the NOELs for the congeneric groups and those for finished mint oils are on the same order of magnitude.
• Criteria for GRAS Status. Based on the above assessment and the application of the scientific judgment of the FEMA Expert Panel, corn mint oil is concluded to be “generally recognized as safe” under conditions of intended use as a flavoring substance. Given the criteria used in the evaluation, recommended specifications should include the following chemical assay: (1) <95% alicyclic secondary alcohols, ketones, and related esters, typically measured as (–)-menthol; (2) <2% 2-isopropylidenecyclohexanones and their metabolites, measured as (–)-pulegone; and (3) <10% monoterpene hydrocarbons, typically measured as limonene.
Safety Assessment of Lemongrass Oil
Lemongrass oil (FEMA No. 2624) is produced by the steam distillation of the freshly cut or slightly dried grasses of Cymbopogon citratus or Cymbopogon flexuosus. The two oils were formerly the main source of natural citral containing upward of 75% of a mixture of neral and geranial in a ratio of approximately 1:4. However, the commercial importance of lemongrass oil has declined as a result of competition from synthetic citral and natural citral from Litsea cubeba oil. C. citratus, the West Indian Type, is produced in Central and South America as well as in Africa and East Asian countries, while C. flexuosus is primarily a product of India.
Lemongrass oil has a lemon-like aroma characteristic of citral. The analytical data cited below are representative of samples of commercial oil intended for use in flavorings. Although consumption of lemongrass as a food in the United States has sharply risen in the past few years, quantitative data on its consumption are not available.
The annual volume of use of lemongrass oil as a flavoring substance has decreased from approximately 5,100 kg in 1975 to 1,470 kg in 1999 (NAS, 1975; Lucas et al., 1999). Based on the most recent data, the daily per capita intake (“eaters only”) is 0.194 mg/person/day (0.0032 mg/kg bw/day).
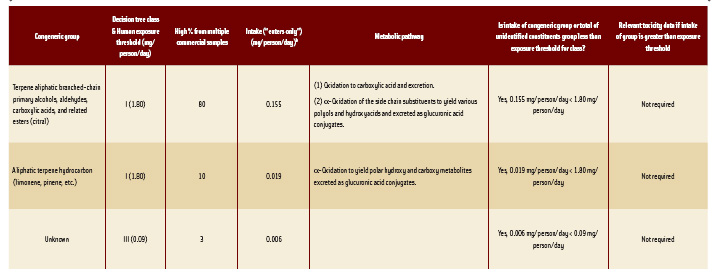
• Principal Congeneric Group. The principal congeneric group in either the West or East Indian types of lemongrass oil is terpene branchedchain primary alcohols (geraniol, nerol, citronellol), aldehydes (citral as a mixture of geranial and neral), acids, and related esters. Analyses of distilled commercial oils reveal that lemongrass oil typically contains 80% of this congeneric group, with citral accounting for the majority.
The biochemical and biological fate of this group of substances has been previously reviewed (JECFA, 2004) and indicates that the major constituents of this congeneric group are effectively detoxicated via oxidation of the corresponding alcohol or aldehyde or ω-oxidation of the branched chain to yield polar polyoxygenated metabolites that are readily excreted either free or in conjugated form (Chadha and Madyastha, 1982; Boyer and Petersen, 1990; Diliberto et al., 1990). The intake of the congeneric group (0.155 mg/person/day) is less than the exposure threshold (1.80 mg/person/day) for Structural Class I. Given the extremely low intake from use of lemongrass oil as a flavoring substance, no toxicity data are required for this congeneric group. Therefore, the intake of this congeneric group from consumption of lemongrass oil is not a safety concern.
In the event that intake of lemongrass oil were to increase significantly (e.g., 20 times the current level of 0.155 mg/person/day for this congeneric group and exceed the human exposure threshold (>1.80 mg/person/day) for Structural Class I, there are numerous subchronic and chronic toxicity and carcinogenicity studies for citral (Hagan et al., 1967; NTP, 2003b), geraniol (Hagan et al., 1967), citronellol (Oser, 1958), and other members of the group (NTP, 1987) that show NOAELs at least three orders of magnitude greater than the hypothetical daily per capita intake (“eaters only”) (3.1 mg/person/day) of this congeneric group resulting from 20 times the current intake of the essential oil.
• Terpene Hydrocarbons. Like corn mint oil, the only other congeneric group that accounts for >2% of the composition of lemongrass oil is a congeneric group of terpene hydrocarbons: (+) limonene, myrcene, etc. This group may account for approximately 10% of lemongrass oil. The intake of terpene hydrocarbons is approximately 0.019 mg/person/day. The metabolism of this group has been discussed previously. The daily per capita intake (0.019 mg/person/day) is approximately 100 times lower than the exposure threshold (1.80 mg/person/day) for Structural Class I. Because intake of this group does not exceed the threshold, no additional data are required. The intake of this congeneric group from consumption of lemongrass oil does not present a safety concern.
--- PAGE BREAK ---
• Unidentified Constituents. The total of unidentified constituents in lemongrass oil is less than 3%. This corresponds to a daily per capita intake (“eaters only”) of up to approximately 0.006 mg/person/day. This intake is lower than the 0.09 mg/person/day threshold for the structural class of highest toxic concern (Class III). Therefore, the total of unidentified constituents does not present a safety concern.
 • Criteria for GRAS Status. In the context of the combined intake of all congeneric groups and the total of unidentified constituents, and any other related data, there is no evidence of any interaction that would present a safety concern. Based on the above assessment and the application of the scientific judgment of the FEMA Expert Panel, lemongrass oil is reaffirmed as “generally recognized as safe” under conditions of intended use as a flavoring substance (Table 6).
• Criteria for GRAS Status. In the context of the combined intake of all congeneric groups and the total of unidentified constituents, and any other related data, there is no evidence of any interaction that would present a safety concern. Based on the above assessment and the application of the scientific judgment of the FEMA Expert Panel, lemongrass oil is reaffirmed as “generally recognized as safe” under conditions of intended use as a flavoring substance (Table 6).
Given the criteria used in the evaluation, recommended specifications should include the following chemical assay: (1) <92% terpene aliphatic branched-chain primary alcohols, aldehydes, carboxylic acids, and related esters, typically measured as citral; and (2) <20% monoterpene hydrocarbons, typically measured as (+)-limonene or myrcene.
FEMA GRAS LISTS Published in Food Technology, in chronological order
Hall, R.L. 1960. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. Food Technol. 14: 488.
Hall, L. and Oser, B.L. 1961. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. II. Food Technol. 15(12): 20.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 3. GRAS substances. Food Technol. 19(2, Part 2): 151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 4. GRAS substances. Food Technol. 24(5): 25-34.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 5. GRAS substances. Food Technol. 26(5): 35-42.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 6. GRAS substances. Food Technol. 27(1): 64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 7. GRAS substances. Food Technol. 27(11): 56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 8. GRAS substances. Food Technol. 28(9): 76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 9. GRAS substances. Food Technol. 29(8): 70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 10. GRAS substances. Food Technol. 31(1): 65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 11. GRAS substances. Food Technol. 32(2): 60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 12. GRAS substances. Food Technol. 33(7): 65-73.
Oser, B.L., Ford, R.A., and Bernard, B.K. 1984. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 13. GRAS substances. Food Technol. 38(10): 66-89.
Oser, B.L., Weil, C.L., Woods, L.A., and Bernard, B.K. 1985. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 14. GRAS substances. Food Technol. 39(11): 108-117.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C., and Newberne, P.M. 1990. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 15. GRAS substances. Food Technol. 44(2): 78,80,82, 84, 86.
--- PAGE BREAK ---
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS substances. Food Technol. 47(6): 104-117.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996a. GRAS flavoring substances 17. Food Technol. 50(10): 72-78, 80-81.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996b. Correction to GRAS flavoring substances 17. Food Technol. 51(2): 32.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1998. GRAS flavoring substances 18. Food Technol. 52(9): 65-66, 68, 70, 72, 74, 76, 79- 92.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1999. Correction to GRAS flavoring substances 18. Food Technol. 53(3): 104.
Newberne, P., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adams, T.B., and Hallagan, J.B. 2000. GRAS flavoring substances 19. Food Technol. 54(6): 66, 68-69, 70, 72-74, 76-84.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Adams, T.B., and McGowen, M.M. 2001. GRAS flavoring substances 20. Food Technol. 55(12): 34-36, 38, 40, 42, 44-55.
Smith, R.I., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, I.J., Portoghese, P.S., Waddell, W.J., Wagner, B.M., And Adams, T.B. 2003. GRAS flavoring substances 21. Food Technol. 57(5): 46-48, 50, 52-54, 56-59.
Corrections & Changes
The chemical name for FEMA No. 2563 in GRAS 3 (Hall and Oser, 1965) was incorrectly listed as 3-hexen-1-ol; the correct name is cis-3-hexen-1-ol.
The chemical name for FEMA No. 2564 in GRAS 3 was incorrectly listed as 2-hexen-1-yl acetate; the correct name is trans-2-hexen-1-yl acetate.
2,2´-(Dithiodimethylene)-difuran (FEMA No. 3146) reported in GRAS 4 (Hall and Oser, 1970) and bis(2-furfuryl) disulfide (FEMA No. 3257) reported in GRAS 5 (Oser and Hall, 1972) are identical materials. Consequently, FEMA No. 3257 has been deleted from the GRAS list.
The chemical name for FEMA No. 3283 in GRAS 5 was incorrectly listed as furfuryl 2-methylbutanoate; the correct name is furfuryl 3-methylbutanoate.
The chemical name for FEMA No. 3353 in GRAS 6 (Oser and Ford, 1973a) was incorrectly listed as 3-hexenyl formate; the correct name is cis-3-hexenyl formate.
The chemical name for FEMA No. 3638 in GRAS 12 (Oser and Ford, 1979) was incorrectly listed as 2-trans-4-cis-7-cis-tridecadienal; the correct name is 2-trans-4-cis-7-cis-tridecatrienal.
The chemical name for FEMA No. 3761 in GRAS 15 (Burdock et al., 1990) was listed incorrectly as 5-methyl-2-hept-4-one; the correct name is 5-methyl-2-hepten-4-one.
The chemical name for FEMA No. 3770 in GRAS 15 was listed incorrectly as 3-oxo-hexanoic acid diglyceride; the correct name is 3-oxo-hexanoic acid glyceride.
The use levels for FEMA 3804, 2-isopropyl-N,2,3-trimethybutyramide, were incomplete as reported in GRAS 17 (Smith et al., 1996a); an average usual use level of 3 ppm and an average maximum use level of 8 ppm in non-alcoholic beverages should also have been listed.
The chemical name for FEMA No. 4054 in GRAS 21 (Smith et al., 2003) was listed incorrectly as 1-menthyl methyl ether; the correct name is l-menthyl methyl ether.
Bernard Wagner retired from the FEMA Expert Panel in October 2003 after a distinguished tenure but will remain an Emeritus member of the Panel.
John Doull retired as a consultant to the Panel in October 2003 but will remain an Emeritus member of the Panel.
Robert L. Smith, Chairman of the FEMA Expert Panel, is Professor, Molecular Toxicology, Imperial College School of Medicine, University of London, South Kensington, London SW7 2AZ, United Kingdom. Other members of the FEMA Expert Panel are Samuel M. Cohen, Professor and Chair, Dept. of Pathology and Microbiology, University of Nebraska Medical Center, Omaha; John Doull, Professor Emeritus, University of Kansas Medical School, Kansas City; Victor J. Feron, TNO Quality of Life, Professor Emeritus, Biological Toxicology, Utrecht University, Zeist, The Netherlands; Jay I. Goodman, Professor, Dept. of Pharmacology and Toxicology, Michigan State University, East Lansing; Lawrence J. Marnett, Dept. of Biochemistry, Center in Molecular Toxicology, School of Medicine, Vanderbilt Institute of Chemical Biology, Nashville, Tenn.; Philip S. Portoghese, Professor, College of Pharmacy, University of Minnesota, Minneapolis; William J.Waddell, Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky; and Bernard M. Wagner, Emeritus Research Professor of Pathology,New York University Medical Center, New York, N.Y. Timothy B. Adams is the Scientific Secretary for the FEMA Expert Panel and Scientific Director of the Flavor and Extract Manufacturers Association, 1620 I St., N.W., Suite 925, Washington, DC 20006. Send reprint requests to author Adams ([email protected]).
References
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1996. The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. Food Chem. Toxicol. 34: 763-828.
Adams, T.B., Doull, J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1997. The FEMA GRAS assessment of furfural used as a flavor ingredient. Food Chem. Toxicol. 35: 739-751.
Adams, T.B., Greer, D.B., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1998. The FEMA GRAS assessment of lactones used as flavor ingredients. Food Chem. Toxicol. 36: 249-278.
Adams, T.B., Cohen, S.M., Doull, J., Feron, V.F., Goodman J.I., Marnett, L.J., Munro, I.C., Portoghese P.S., Smith, R.L., Waddell W.J., and Wagner, B.M. 2004. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 42: 157-185.
Austin, C.A., Shephard, E.A., Pike, S.F., Rabin, B.R., and Phillips, I.R. 1997. The effect of terpenoid compounds on cytochrome P-450 levels in rat liver. Biochem. Pharmacol. 37: 2223-2229.
Boyer, C.S. and Petersen, D.R. 1990. The metabolism of 3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and cytosolic fractions. Drug Metabol. Dispos. 18: 81-86.
Chadha, A. and Madyastha, K.M. 1982. Omega-hydroxylation of acyclic mono-terpene alcohols by rat lung microsomes. Biochemical and Biophysical Research Communications, 108: 1271-1277.
Chen, L., Lebetkin, E. H., and Burka, L.T. 2001. Metabolism of (R)-(+)-pulegone in F344 rats. Drug Metabol. Dispos. 29: 1567-1577.
Cramer, G.M., Ford, R.A., and Hall, R.L. 1978. Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol. 16: 225-276.
Crowell, P., Elson, C.E., Bailey, H., Elegbede, A., Haag, J., and Gould, M. 1994. Human metabolism of the experimental cancer therapeutic agent d-limonene. Cancer Chemother Pharmacol. 35: 31-37.
DeOliveira, A., Ribeiro-Pinto, L., Otto, S., Goncalves A., and Paumgartten F. 1997. Induction of liver monooxygenase by β-myrcene. Toxicology. 124: 135-140.
Devries, H. and Stuiver, M. 1961. In “Sensory Communication,” ed. W.A. Rosenblith, p. 159. Wiley, New York.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T., and Birnbaum, L.S. 1990. Metabolism of citral, an α,β-unsaturated aldehyde, in male F344 rats. Drug Metabol. Dispos. 18: 866-875.
Eriksson, K. and Levin, J.O. 1996. Gas chromatographic mass spectrometric identification of metabolites from alphapinene in human urine after occupational exposure to sawing fumes. J. Chromatog.677(1): 85-98.
FCC. 2003. “Food Chemicals Codex,” 5th ed. National Academy Press, Washington, D.C.
Florin, I., Rutberg L., Curvall M., and Enzell C.R. 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames test. Toxicology 18: 219-232.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., and Taylor, J.M. 1967. Food flavourings and compounds of related structure. II. Sub-acute and chronic toxicity. Food Cosmet. Toxicol. 5: 141-157.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., and Curren, R.D. 1989. An evaluation of food flavoring ingredients in a genetic toxicity screening battery. Toxicologist. 9(1): 257.
Hoechst AG. 1991. Unveröffentl. Unters. (Ber.-Nr. 91.0246). Private communication to FEMA. Unpublished report. Submitted to WHO by Flavor and Extract Manufacturers Association of the United States, Washington, D.C.
Ishida, T., Asakawa, Y., Takemoto, T., and Aratani, T. 1981. Terpenoids biotransformation in mammals III: Biotransformation of alpha-pinene, beta-pinene, 3-carene, carane, myrcene, and p-cymene in rabbits. J. Pharm. Sci. 70: 406-415.
JECFA. 1997. Evaluation of certain food additives and contaminants. 46th Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Tech. Rept. Series 868. World Health Org., Geneva.
JECFA. 1999. Safety evaluation of certain food additives. 51st Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Food Additives Series 42. World Health Org., Geneva.
JECFA. 2004. Safety evaluation of certain food additives. 61st Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Food Additives Series 52. World Health Org. , Geneva.
Kodama, R., Yano, T., Furukawa, K., Noda, K., and Ide, H. 1976. Studies on the metabolism of d-limonene. IV. Isolation and characterization of new metabolites and species differences. Metabolism 6: 377-389.
Lehman-McKeeman, L.D. and Caudill, D. 1999. Development of an in vitro competitive binding assay to predict alpha 2u-globulin nephropathy. Vitr. Mol. Toxicol. 12(2): 83-95.
Lucas, C.D., Putnam, J.M., and Hallagan, J.B. 1999. Flavor and Extract Manufacturers Assocaition (FEMA) of the United States 1995 poundage and Technical Effects Update Survey. Washington D.C.Self-published.
Madsen, C., Wurtzen, G., and Carstensen, J. 1986. Short-term toxicity in rats dosed with menthone. Toxicol. Lett. 32: 147-152.
Madyastha, K.M. and Srivatsan, V. 1987. Metabolism of beta-myrcene in vivo and in vitro: Its effects on rat-liver microsomal enzymes. Xenobiotica. 17: 539-549.
Madyastha, K.M. and Srivatsan, V. 1988. Studies on the metabolism of l-menthol in rats. Drug Metab. Dispos. 16: 765.
Maltzman, T., Christou, M., Gould, M., and Jefcoate, R. 1991. Effects of monoterpenoids on in vivo DMBA-DNA adduct formation and on phase I hepatic metabolizing enzymes. Carcinogenesis 12: 2081-2087.
McClanahan, R.H., Thomassen, D., Slattery J.T., and Nelson, S.D. 1989. Metabolic activation of (R)-(+)-pulegone to a reactive enonal that covalently binds to mouse liver proteins. Chem. Res. Toxicol. 2: 349-355.
Miyazawa, M., Shindo, M., and Shimada, T. 2002. Sex differences in the metabolism of (+)- and (–)-limonene enantiomers to carveol and perillyl alcohol derivatives by cytochrome P450 enzymes in rat liver microsomes. Chem. Res. Toxicol. 15(1): 15-20.
Muller, W. 1993. Evaluation of mutagencity testing with Salmonella typhimurium TA102 in three different laboratories. Environ. Health Perspec. Suppl. 101: 33-36.
Munro, I.C., Ford, R.A., Kennepohl E., and Sprenger, J.G., 1996. Thresholds of toxicological concern based on structure-activity relationships. Drug Metab. Rev. 28(1/2): 209-217.
NAS. 1975. ”Evaluating the Safety of Food Chemicals.” Natl. Acad. of Sciences Washington, D.C.
NCI. 1979. Bioassay of dl-menthol for possible carcinogenicity. U.S. Dept. of Health, Education and Welfare. Natl. Tech. Rept. Series 98. Natl. Cancer Inst., Bethesda, Md.
Newberne, P.M., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Lucas, C.D., and Ford, R.A. 1999. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 37: 789-811.
Nijssen, B., van Ingen-Visscher, K., and Donders, J., 2003. Volatile compounds in food. Centraal Instituut Voor Voedingsonderzioek TNO. Zeist, The Netherlands. www.voeding.tno.nl/vcf/VcfNavigate.cfm.
NTP. 1987. Carcinogenesis studies of food grade geranyl acetate (71% geranyl acetate, 29% citronellyl acetate) (CAS No. 105-87-3) in F344/N rats and B6C3F1 mice (gavage studies). NTP-TR-252; PB-88-2508. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1990. Carcinogencity and toxicology studies of d-limonene in F344/N rats and B6C3F1 mice. NTPTR-347. U.S. Dept of Health and Human Services. NIH Pub. 90-2802. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 2002. Toxicity studies of pulegone in B6C3F1 mice and rats (gavage studies). Battelle Research Laboratories, Study G004164-X. Unpublished report. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 2003a. Draft report on the initial study results from a 90-day toxicity study on beta-myrcene in mice and rats. Study C99023 and A06528. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 2003b. Report on the toxicology and carcinogenesis studies of citral (microencapsulated) (CAS No. 5392-40-5) in F344/N rats and B6C3F1 mice (feed studies). Tech. Rept. Series 505. Natl. Toxicology Program, Research Triangle Park, N.C.
Oser, O.M. 1958. Toxicological screening of components of food flavours. Class VI. Citronellol and linalool. Trubek Laboratories, Inc., East Rutherford, N.J.
Poon, G., Vigushin, D., Griggs, L.J., Rowlands, M.G., Coombes, R.C., and Jarman, M. 1996. Identification and characterization of limonene metabolites in patients with advanced cancer by liquid chromatography/mass spectrometry. Drug Metab. Dispos. 24: 565-571.
Rivedal, E., Mikalsen, S.O., and Sanner, T. 2000. Morphological transformation and effect on gap junction intercellular communication in Syrian hamster embryo cells as screening tests for carcinogens devoid of mutagenic activity. Toxic. In Vitro 14: 185-192.
Sasaki,Y.F., Imanishi,, H., Ohta,, T., and Shirasu, Y. 1989. Modifying effects of components of plant essence on the induction of sister-chromatid exchanges in cultured Chinese hamster ovary cells. Mutat. Res. 226: 103-110.
Serota, D. 1990. 28-Day toxicity study in rats. HLA study 642-477. Private communication to FEMA. Unpublished rept. Hazelton Laboratories America, Rockville, MD.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2002a. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. 40: 429-451.
Smith, R.L., Adams, T.B., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Portoghese P.S., Waddell, W.J., Wagner, B.M., Rogers, A.E., Caldwell, J., and Sipes, IG. 2002b. Safety assessment of allylalkoxybenzene derivativesused as flavouring substances—methyleugenol and estragole. Food Chem. Toxicol. 40: 851-870.
Smith, R.L., Cohen, S., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J.,.Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2004. Safety evaluation of natural flavour complexes. Toxicol. Lett. 149: 197-207.
Smith, R.L., Cohen, S.M., Doull, J., Feron, V.J., Goodman J.I., Marnett L.J., Portoghese P.S., Waddell, W.J., Wagner B.M., Hall, R.L., Higley, N.A., Lucas-Gavin, C., and Adams, T.B. 2005a. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 43: 345-363.
Smith, R.L., Cohen, S., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J.,.Munro I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2005b. Criteria for the safety evaluation of flavoring substances. Food Chem. Toxicol. 43: 1141-1177
Splindler, P. and Madsen, C. 1992 Subchronic toxicity study of peppermint oil in rats. Toxicol. Lett. 62: 215-220.
Swenberg, J. and Schoonhoven, R. 2002. Private communication to FEMA. University of North Carolina, Chapel Hill.
Thomassen, D., Knebel, N., Slattery, J.T., McClanahan, R. H., and Nelson, S.D. 1992. Reactive intermediates in the oxidation of menthofuran by cytochrome P-450. Chem. Res. Toxicol. 5: 123-130.
Vigushin, D., Poon, G.K., Boddy, A., English, J., Halbert, G.W., Pagonis, C., Jarman, M., and Coombes, R.C. 1998. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Chemother. Pharmacol. 42(2): 111-117.
Williams, R.T. 1940. Studies in detoxication. 7. The biological reduction of l-menthone to d-neomenthol and of d-isomenthone to d-isomenthol in the rabbit. The conjugation of d-neomenthol with glucuronic acid. Biochem. J. 34: 690-697.
Woods. L.A. and Doull, J. 1991. GRAS evaluation of flavoring substances by the Expert Panel of FEMA. Regulat. Toxicol. Pharmacol. 14: 48-58.
Yamaguchi, T., Caldwell, J., and Farmer, P.B. 1994. Metabolic fate of [3H]-l-menthol in the rat. Drug Metab. Dispo. 22: 616-624.
Zamith, H.P., Vidal, M.N.P., Speit, G., and Paumgartten, F.J.R. 1993. Absence of genotoxic activity of beta-myrcene in the in vivo cytogenetic bone marrow assay. Brazilian J. Med. Biol. Res. 26: 93-98.
