Lignans in Food and Nutrition
Plant lignans in high-fiber foods are precursors of mammalian lignans, which are associated with a reduced risk of cardiovascular disease and cancer.
Dietary fiber has been recognized as a contributing factor in human health for more than 50 years (Hipsley, 1953). Building on the pioneering work of Burkitt and Trowell (1975) in East Africa in the early 1970s, medical researchers began to recognize that high levels of fiber in the diet play a significant role in offsetting chronic diseases such as diabetes, cardiovascular disease, and certain cancers. Even at that early stage, dietary fiber was recognized to be the remnants of plant cell walls that were resistant to digestion, including cellulose, hemicelluloses, lignin, and polysaccharides such as pectin (DeVries et al., 1999).
 Extensive research in the years following these discoveries has confirmed the protective role of dietary fiber. Medical and nutrition researchers have examined the role of various components of dietary fiber in an effort to understand the mechanism(s) of action. Dietary fiber, which is insoluble in aqueous conditions, is thought to bind toxins, increase fecal bulk, and reduce the severity of constipation and diverticular disease.
Extensive research in the years following these discoveries has confirmed the protective role of dietary fiber. Medical and nutrition researchers have examined the role of various components of dietary fiber in an effort to understand the mechanism(s) of action. Dietary fiber, which is insoluble in aqueous conditions, is thought to bind toxins, increase fecal bulk, and reduce the severity of constipation and diverticular disease.
The chemical components of insoluble fiber include cellulose, lignin, and most hemicelluloses found in the primary and secondary cell walls of plants. The cellulose in cell walls is chemically bonded to hemicelluloses and lignin (Salisbury and Ross, 1978). The latter two polymers act to give significant structural rigidity and resistance to the action of enzymes and acids. Thus, insoluble fiber is poorly fermented by the bacteria in the colon.
Soluble fiber, such as pectin, β-glucans, and some hemicelluloses, are found in the primary cell walls and the space between adjacent cell walls, called the middle lamella. Soluble fiber acts to increase the viscosity of gastrointestinal fluid, which slows the digestion of starch and the transport of glucose. It is extensively fermented by bacteria in the large intestine. Thus, soluble fiber provides fuel for the colonic bacteria, which in turn produce the short-chain fatty acids that are the primary source of fuel for the cells lining the colon (Livesey and Elia, 1995). It is becoming increasingly clear that the action of colonic bacteria on dietary fiber produces a number of physiologically active molecules.
 Lignans Are Different from Lignins
Lignans Are Different from Lignins
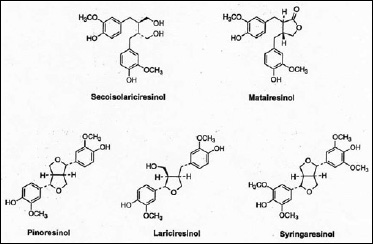
More recently, some of the minor components associated with dietary fiber have been isolated and found to produce important physiological effects. Of significant interest are the lignans, a group of relatively simple di-phenols, which share the same molecular origins as the much more complex polymeric lignins. However, the biosynthetic pathway to the lignans diverges from lignin at the early stage of the phenylpropenols, primarily coniferyl and sinapyl alcohols. Substantial evidence now indicates that the biosynthetic pathways to the lignans and lignins are fully independent after this stage (Lewis et al., 1998). All lignans share 2,3-dibenzylbutane as their common chemical backbone (Fig. 1).
In plants, lignans function as defensive chemicals, protecting them from attack by insects, microorganisms, and even other plants (Ayres and Loike, 1990). So it is not surprising that lignans are associated with plant cell wall material, especially the outermost layers of cells. It has been shown, for example, that lignans in cereal grains are concentrated in the outermost pericarp layer of cells, followed by the aleurone layer, and are therefore abundant in cereal brans, a rich source of dietary fiber (Glitso et al., 2000). At present, however, there is no evidence that lignans are chemically linked to plant cell wall components, such as lignin, even though they share a common biosynthetic origin. It is therefore possible to isolate intact lignans from plant material by solvent extraction and other chemical means.
--- PAGE BREAK ---
 Plant Lignans Are Precursors of Mammalian Lignans
Plant Lignans Are Precursors of Mammalian Lignans
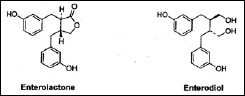
Following ingestion, the lignans in plant-based foods—primarily oilseeds, cereal grains, vegetables, fruits, and legumes—are converted by bacteria in the large intestine into two simple diphenols: enterolactone and enterodiol (Fig. 2) (Stitch et al., 1980; Setchell et al., 1980). These compounds are called mammalian lignans because they have been found only in mammals. They are formed by the enzymatic removal of methyl and hydroxyl groups from the plant lignans.
Once formed, the two compounds are absorbed from the intestine and undergo enterohepatic circulation. Further processing results in conjugates being formed in the liver, which are subsequently excreted in the bile and reabsorbed from the intestine. Ultimately, both enterolactone and enterodiol are excreted in the urine as glucuronate and sulfate conjugates and in the feces as free phenols (Adlercreutz et al., 1995). Higher intake of dietary plant lignans is associated with higher levels of enterolactone in blood serum (Kilkkinen et al., 2003). Thus, lignans may serve as biomarkers of a healthy high-fiber diet containing whole grains, fruits, and vegetables (Lampe, 2003).
Mammalian lignans have been found to exhibit a number of significant physiological effects (Tham, et al., 1998). Enterolactone is a moderate inhibitor of estrogen synthetase (aromatase) and lowers estrogen levels, while enterodiol is a weaker inhibitor (Makela et al., 2000). Both compounds also increase the levels of sex hormone–binding globulin (SHBG), which is important in controlling the availability of androgens and estrogens in the body. Plant lignans are therefore considered to be one of several chemical classes of phytoestrogens. Lignans are also effective as antioxidants and may inhibit lipid peroxidation. Enterolactone decreases the plasma levels of F2-isoprostanes, a measure of lipid peroxidation (Vanharanta et al., 2002).
A number of epidemiological studies have found an inverse association between dietary intake of lignans and the risk of cardiovascular disease and certain types of cancer (Arts and Hollman, 2005). The studies have utilized both the concentrations of enterolactone and enterodiol found in blood serum and urine and the estimated dietary intake of two common plant lignans: matairesinol and secoisolariciresinol.
Vanharanta et al. (1999) found a 65% lower risk of acute coronary events for men with high serum concentrations of enterolactone. van der Schouw et al. (2005) found that a diet high in plant lignans both increased and decreased the levels of certain risk factors for cardiovascular disease in men.
The effects of lignans on cancer have been studied more thoroughly (Arts and Hollman, 2005). Several studies have found an inverse association between lignan levels and the risk of breast cancer. For example, an inverse association was found for breast cancer in premenopausal women, but not postmenopausal women, in the U.S. (McCann et al., 2002). Protective associations have also been reported for endometrial, ovarian, and thyroid cancer in women (Arts and Hollman, 2005). Possible protective roles in prostate and colon cancer require further study. However, in a recent in-vitro study, both enterolactone and enterodiol caused dose- and time-dependent decreases in the number of human colon cancer cells (Qu et al., 2005).
 Plant Lignans in Food
Plant Lignans in Food
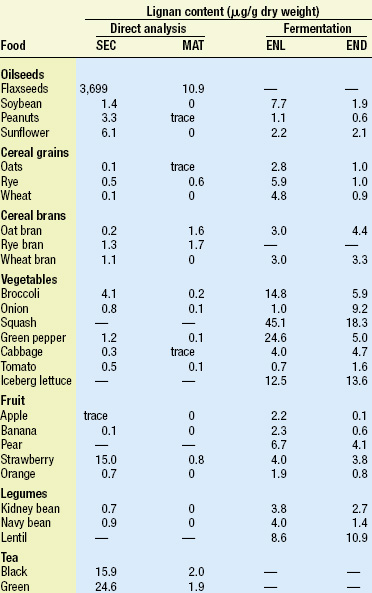
Lignans occur in a wide variety of plant-based foods. Table 1, adapted from Meagher and Beecher (2000), compares the levels of lignans found in many foods based on two different methods of analysis. The direct method is based on the work of Mazur et al. (1996, 1998a, b), who analyzed the content of two common plant lignans, secoisolariciresinol (SEC) and matairesinol (MAT), using isotope dilution gas chromatographic–mass spectrometric analysis of food extracts. The indirect method is based on the earlier work of Thompson et al. (1991), who used extraction and capillary gas chromatography to analyze the amount of enterolactone (ENL) and enterodiol (END) produced by in-vitro fermentation of various foods with human fecal microbiota. The latter results are in good agreement with the levels found in urine.
Flaxseed contains by far the highest level of lignans, but vegetables, cereal brans, and legumes are also good sources, based on the amounts consumed in a high-fiber diet. Tea is also a good source of lignans. A useful summary of phytoestrogen concentrations estimated in foods can be found in de Kleijn et al. (2001).
One of the greatest issues still facing researchers in this field is the limited information on the form and levels of plant lignans in food. This is best illustrated by comparing the levels found for SEC and MAT in foods with the levels of ENL and END produced by in-vitro fermentation, as shown in Table 1. In most cases, far higher levels of the mammalian lignans are produced by bacterial fermentation of food than would be predicted by the levels of SEC and MAT in food.
--- PAGE BREAK ---
Until fairly recently, it was assumed that SEC and MAT were the only sources of mammalian lignans in food. Recent studies by Heinonen et al. (2001) now show that other plant lignans are also important sources of the mammalian lignans. Pinoresinol, lariciresinol, syringaresinol, and others were shown to be precursors of ENL and END. These three plant lignans occur in rye bran in amounts 10–50 times higher than the amounts of SEC and MAT and can produce 10–12 times the amount of ENL.
Research on the extraction of plant lignans from food also demonstrates that alkaline hydrolysis of crude extracts dramatically increases the yields of most lignans (Milder et al., 2004). This step is apparently necessary to free the lignans from a variety of chemical forms present in food. Plant lignans generally occur in foods as glucosides. In addition, two studies have shown that the glucoside of SEC exists as oligomeric esters of hydroxymethyl glutaric acid in flaxseed (Ford et al., 2001; Kanal-Eldin et al., 2001). There are no reports on the oligomeric structures of other plant lignans in food, but they are likely formed by enzyme-mediated coupling of the diphenols. Thus, alkaline hydrolysis, followed by enzymatic removal of glucose, is necessary to free the lignans for complete extraction and analysis.
Lignans may also occur in different forms in different foods. For example, omitting the alkaline hydrolysis step decreases the yield of SEC from flaxseed by 81%, and from broccoli by 2–22%, while the yield from tea is unchanged (Milder et al., 2004). This suggests that lignans may occur as monomers in tea, as a mixture of monomers and oligomers in broccoli, and predominantly as oligomers in flaxseed.
Other foods may also contain a range of monomers and oligomers, which could affect accessibility for bacterial fermentation. Glitso et al. (2000), however, showed that lignans in the pericarp layer of cells are readily converted to mammalian lignans in pigs despite the very low degradability of this dietary fiber. Thus, plant lignans are not inextricably bound to dietary fiber. Also, Begum et al. (2004) showed that enterolactone was formed in rats from wheat bran and rye bran from which the plant lignans had previously been extracted. This suggests that plant cell wall lignins may also serve as a source of mammalian lignans. Should these results be confirmed in humans, it opens the possibility that mammalian lignans may be formed in greater amounts and in greater numbers of physiologically active compounds than previously thought.
Relatively little has been reported on the effects of food processing on plant lignans. Meagher and Beecher (2000) summarized the short list of publications that have examined the lignan content of breads, crackers, and breakfast cereals by either the direct or indirect method of analysis. It is not clear if processing affected lignan content. Matairesinol (MAT) has been shown to be sensitive to aqueous alkaline conditions. Recovery of a known amount of MAT added to bread was only about 50% following extraction by alkaline hydrolysis (Milder et al., 2004). Baking processes using alkaline leavening agents could therefore reduce the content of MAT in whole-grain breads (Jenkins, 1995). However, Nesbitt et al. (1999) showed that there was no difference in urinary excretion of lignans following the ingestion of raw flaxseed, or flaxseed incorporated into processed muffins and bread. The vast majority of the lignans in flaxseed is SEC, which may be more stable to processing conditions.
Looking Forward
Clearly, more research is needed to fully understand the different forms of lignans in food and their conversion to mammalian lignans. Effects of food processing on plant lignans should receive greater attention. It is also very important to determine if there are additional precursors of mammalian lignans, and how this might affect nutrition studies. Based on their effects in modulating steroid hormone metabolism, cell growth, and lipid peroxidation, the lignans may eventually be proven to exert protective roles in other human and animal diseases.
The author, a Professional Member of IFT, is Consultant in Food and Nutrition Chemistry and Visiting Lecturer at Framingham State College. Beginning in fall 2005, he will be Visiting Lecturer at Harvard School of Public Health, Boston, Mass., and Associate Professor, Dept. of Chemistry and Food Science, Framingham State College, Framingham, MA 01701 ([email protected]).
References
Adlercreutz, H., van der Wilelt, J., Kinzel, J., Attalla. H., Wahala, K., Makela, T., Hase, T., and Fotsis, T. 1995. Lignan and isoflavonoid conjugates in human urine. J. Steroid Biochem. Molec. Biol. 52: 97-103.
Arts, I.C.W. and Hollman, P.C.H. 2005. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 81 (Suppl): 317S-325S.
Ayres, D.C. and Loike, J.D. 1990. "Lignans: Chemical, Biological, and Clinical Properties." Cambridge University Press, Cambridge.
Begum, A.N., Nicolle, C., Mila, I., Lapierre, C., Nagano, K., Fukushima, K., Heinonen, S-M., Adlercreutz, H., Remesy, C., and Scalbert, A. 2004. Dietary lignins are precursors of mammalian lignans in rats. J. Nutr. 134: 120-127.
Burkitt, D.P. and Trowell, H.C. 1975. "Refined Carbohydrate Foods and Disease. Some Implications for Dietary Fiber. " Academic Press, London.
de Kleijn, M.J.J., van der Schouw, Y.T., Wilson, P.W.F., Adlercreutz, H., Mazur, W., Grobbee, D.E., and Jacques, P.F. 2001. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: The Framingham Study. J. Nutr. 131: 1826-1832.
DeVries, J.W., Prosky, L., Li, B., and Cho, S. 1999. A historical perspective on defining dietary fiber. Cereal Foods World 44: 367-369.
Ford, J.D., Huang, K-S., Wang, H-B., Davin, L.B., and Lewis, N.G. 2001. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed. J. Natural Prod. 64: 1388-1397.
Glitso, L.V., Mazur, W.M., Adlercreutz, H., Wahala, K., Makela, T., Sandstrom, B., and Bach Knudsen, K.E. 2000. Intestinal metabolism of rye lignans in pigs. Brit. J. Nutr. 84: 429-437.
Heinonen, S., Nutmi. T., Liukkonen, K., Poutanen, K., Wahala, K., Deyama, T., Nishibe, S., and Adlercreutz, H. 2001. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 49: 3178-3186.
Hipsley, E.H. 1953. Dietary "fibre" and pregnancy toxaemia. Brit. Med. J. 2: 420-422.
Jenkins, D.J.A. 1995. Incorporation of flaxseed or flaxseed components into cereal foods. In "Flaxseed in Human Nutrition," ed. S. Cunnane and L.U. Thompson, pp. 281-294. AOAC Press, Champaign, Ill.
Kanal-Eldin, A., Peerlkamp, N., Johnsson, P., Andersson, R., Andersson, R.E., Lundgren, L.N., and Aman, P. 2001. An oligomer from flaxseed composed of secoisolariciresinoldiglucoside and 3-hydroxy-3-methylglutaric acid residues. Phytochem. 58: 587-590.
Kilkkinen, A., Valsta, L.M., Virtamo, J., Stumpf, K., Adlercreutz, H., and Pietinen, P. 2003. Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J. Nutr. 133: 1830-1833.
Lampe, J.W. 2003. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J. Nutr. 133 (Suppl. 3): 956S-964S.
Lewis, N.G., Davin, L.B., and Sarkanen, S. 1998. Lignin and lignan biosynthesis: Distinctions and reconciliations. Chpt. 1 in "Lignin and Lignan Biosynthesis," eds. N.G. Lewis and S. Sarkanen, pp. 1-27. Am. Chem. Soc., Washington, D.C.
Livesey, G. and Elia, M. 1995. Short-chain fatty acids as an energy source in the colon: Metabolism and clinical implications. In "Physiological and Clinical Aspects of Short-Chain Fatty Acids," ed. J. Cummings, J. Rombeau, and T. Sakata, pp. 427-481. Cambridge University Press, Cambridge.
Makela, T.H., Wahala, K.T., and Hase, T.A. 2000. Synthesis of enterolactone and enterodiol precursors as potential inhibitors of human estrogen synthetase (aromatase). Steroids 65: 437-441.
Mazur, W.M., Fotsis, T., Wahala, K., Ojala, S., Salakka, A., and Adlercreutz, H. 1996. Isotope-dilution gas chromatographic–mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal. Biochem. 233: 169-180.
Mazur, W.M., Duke, J.A., Wahala, K., Rasku, S., and Adlercreutz, H. 1998a. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 9: 193-200.
Mazur, W.M., Wahala, K., Rasku, S., Salakka, A., Hase, T., and Adlercreutz, H. 1998b. Lignan and isoflavonoid concentrations in tea and coffee. Brit. J. Nutr. 79: 37-45.
McCann, S.E., Moysich, K.B., Freudenheim, J.L., Ambrosone, C.B., and Shields, P.G. 2002. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J. Nutr. 132: 3036-3041.
Meagher, L.P. and Beecher, G.R. 2000. Assessment of data on the lignan content of foods. J. Food Compos. Anal. 13: 935-947.
Milder, I.E.J., Arts, I.C.W., Venema, D.P., Lasaroms, J.J.P., Wahala, K., and Hollman, P.C.H. 2004. Optimization of a liquid chromatography–tandem mass spectrometry method for quantification of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in foods. J Agric. Food Chem. 52: 4643-4651.
Nesbitt, P.D., Lam, Y., and Thompson, L. U. 1999. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 69: 549-555.
Qu, H., Madl, R.L., Takemoto, D.J., Baybutt, R.C., and Wang, W. 2005. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J. Nutr. 135: 598-602.
Salisbury, F.B and Ross, C.W. 1978. "Plant Physiology," 2nd ed. Wadsworth Publishing Co., Belmont, Calif.
Setchell, K.D.R., Lawson, A.M., Mitchell, F.L., Adlercreutz, H., Kirk, D.N., and Axelson, M. 1980. Lignans in man and in animal species. Nature 287: 740-742.
Stitch, S.R., Toumba, J.K., Groen, M.B., Funke, C.W., Leemhuis, J., Vink, J., and Woods, G. 1980. Excretion, isolation, and structure of a new phenolic constituent of female urine. Nature 287: 738-740.
Tham, D.T., Gardner, C.D., and Haskell, W.L. 1998. Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 83: 2223-2235.
Thompson, L.U., Robb, P., Serraino, M., and Cheung, F. 1991. Mammalian lignan production from various foods. Nutr. Cancer 16: 43-52.
van der Schouw, Y.T., Sampson, L., Willett, W.C., and Rimm, E.B. 2005. The usual intake of lignans but not that of isoflavones may be related to cardiovascular risk factors in U.S. men. J. Nutr. 135: 260-266.
Vanharanta, M., Voutilainen, S., Lakka, T.A., van der Lee, M., Adlercreutz, H., and Salonen, J.T. 1999. Risk of acute coronary events according to serum concentrations of enterolactone: A prospective population-based case-control study. Lancet 354: 2112-2115.
Vanharanta, M., Voutilainen, Nurmi, T., Kaikkonen, J., Roberts, L.J., Morrow, J.D., Adlercreutz, H., and Salonen. 2002. Association between low serum enterolactone and increased plasma F2-isoprostanes, a measure of lipid peroxidation. Atherosclerosis 160: 465-469.
