Controlling Listeria In The Food Processing Environment
Control of Listeria in the processing plant is an unending task requiring careful thought, vigilance in observation and sampling, diligence in tracking, and appropriate corrective action.
Listeria monocytogenes causes human listeriosis. Listeriosis can exist in two forms: a self-limiting gastroenteritis and invasive listeriosis that can be life-threatening. The gastrointestinal form is characterized by flu-like symptoms (e.g., diarrhea, vomiting, and fever) that occur 9–48 hr after ingestion of contaminated food. In contrast, invasive listeriosis may have an onset time of 2–6 weeks, and adults may experience septicemia, meningitis, and endocarditis, whereas unborn fetuses may develop abscesses in their liver, lungs, and other organs, often resulting in spontaneous abortion and stillbirth. Surviving children may be seriously ill with meningitis and neurological impairment (CDC, 2001).
 Foodborne listeriosis causes approximately 2,500 cases, including 500 deaths, annually in the United States, costing an estimated $2.33 billion, making listeriosis the second most costly foodborne illness after salmonellosis (Buzby and Roberts, 1996). Consequently, foodborne listeriosis has been targeted for reduction by many public heath programs, most notably Healthy People 2010—a comprehensive nationwide health promotion and disease prevention program developed by the U.S. Dept. of Health and Human Services to reduce bacterial infections and enhance life expectancy and quality (www.healthypeople.gov).
Foodborne listeriosis causes approximately 2,500 cases, including 500 deaths, annually in the United States, costing an estimated $2.33 billion, making listeriosis the second most costly foodborne illness after salmonellosis (Buzby and Roberts, 1996). Consequently, foodborne listeriosis has been targeted for reduction by many public heath programs, most notably Healthy People 2010—a comprehensive nationwide health promotion and disease prevention program developed by the U.S. Dept. of Health and Human Services to reduce bacterial infections and enhance life expectancy and quality (www.healthypeople.gov).
Recontamination from the processing environment is the principal source of Listeria contamination of processed ready-to-eat foods (Tompkin, 2002). Scientists, regulators, and food processors have striven for the past two decades to seek out and control L. monocytogenes in such environments. Progress was evidenced by a steady decline in listeriosis cases from 1996 to 2001 (www.cdc.gov/foodnet/annual/2003/2003_report.pdf).
Despite enormous efforts, costs, and early successes by the industry, some believe it is not possible to eradicate this organism from the food processing environment with current available technology (Tompkin, 1999). The challenge of controlling this organism is reflected by a leveling-off of the incidence of foodborne listeriosis in 2001 and 2002 (CDC, 2004). Consequently, the food industry must remain vigilant in its Listeria control efforts.
Control is unlikely without the ability to first find the organism. The following discussion assumes that the plant’s Hazard Analysis Critical Control Point (HACCP) plan has been appropriately validated and verified and Good Manufacturing Practices (GMPs) are being followed.
Where to Look
Factory environments are not sterile. L. monocytogenes is very widespread in the natural environment and likely to be reintroduced into food production facilities (Tompkin, 2002). The bacterium also has properties permitting it to survive and effectively compete with other microbes in food processing environments. Its ability to grow at refrigeration temperatures gives it a competitive advantage over non-psychrotrophic microbes. It is also resistant to freezing and high salt content and can adapt to stresses that exist at times in food production facilities. Some harsh conditions in the factory environment (e.g., acidity) can result in cross-protection of Listeria to other stresses (e.g., heat) (Lou and Yousef, 1999).
The areas of greatest relative risk for contaminating food in the factory environment are where Listeria has grown to high numbers. These growth niches must be sought and eliminated when found. Areas where water, food (for microbial growth), and time (e.g., areas not accessible for cleaning) combine at a suitable temperature to produce microbial growth niches. The best places to sample for Listeria are those high-moisture environments where the organism has had opportunity to incubate.
--- PAGE BREAK ---
It is important to investigate and control conditions that create or transmit Listeria niches. Elimination of all known Listeria niches is important, but the greatest immediate risk of product contamination is from such niches and unsanitary conditions occurring after a validated critical control point (CCP).
 Unsanitary operating and maintenance/repair practices and unsanitary equipment/facility design may transmit and/or create Listeria growth niches in the factory environment. Selected examples are described in Table 1.
Unsanitary operating and maintenance/repair practices and unsanitary equipment/facility design may transmit and/or create Listeria growth niches in the factory environment. Selected examples are described in Table 1.
In-Plant Risk Assessment
A number of food processors have found it helpful to break down their routine environmental sampling program into four zones (ICMSF, 2002). Zone 1 samples are those taken from direct and indirect (e.g., overhead pipes) product-contact areas. Zone 2 samples are surfaces adjacent to Zone 1 and include areas like equipment framework and guards. Zone 3 includes surfaces in ready-to-eat (RTE) product zones such as floors, drains, walls, and equipment. Zone 4 areas are more remote from RTE product zones and include such locations as warehouses, loading docks, employee break rooms, and locker rooms. Rotating sampling sites at an appropriate frequency will result in more-comprehensive sampling of the factory environment.
The probability of RTE product contamination is affected by a number of variables, including but not limited to (a) proximity of microbial growth niches to the product stream, (b) number of growth niches, (c) spatial relationship of niches to product stream, (d) microbial populations in niches, (e) extent of niche disruption, and (f) exposure of product stream to the environment (Faust and Gabis, 1988).
Our investigational approach has been to categorize in-factory observations and sampling into regions of "high," "medium," and "indirect" relative risk of product contamination. High-risk areas are somewhat analogous to Zone 1. These exist where moist, entrapped (or standing) residues are located close to the product stream.
Indirect-risk samples would include those from Zones 2–4 that do not produce an obvious direct risk of product contamination. However, the microbial ecology of food processing environments is so dynamic that one does not always readily observe the true risk.
Medium-risk areas are similar to high-risk areas but exist before a likely microbicidal step. These usually require a study to determine the lethality and risk. These types of studies may result in discovery of a previously unknown CCP.
Medium-risk areas may also be areas or practices which might result in contamination of the product infrequently.
Environmental Sampling
Environmental sampling sites may include pre-operational (post-sanitation) product-contact surfaces, and operational and post-operational factory surfaces.
• Pre-Operational Samples. Pre-operational samples are useful to verify effective sanitation of food-contact surfaces. The U.S. Dept. of Agriculture indicated that suitable indicator tests such as "Listeria-like" organisms or "Listeria species" can be used (USDA, 2003). This approach encourages factories to aggressively sample Zone 1 surfaces, document effective sanitation, and take corrective action in the event of positive data.
--- PAGE BREAK ---
It is prudent to test finished product for the presence of L. monocytogenes using an appropriate statistically based sampling plan, if such indicators are found. In this event, an in-factory risk assessment and hold-and-test product sampling would be warranted until microbial control is regained.
Indicators of sanitation efficacy can also include the aerobic plate count (APC) and coliform count. However, these should not be relied on as pathogen indicators (Kornacki and Johnson, 2001).
• Operational or Post-Operational Samples. Food processing plant environments are dynamic, with variations in activity, including traffic patterns, cleaning steps, processing steps and times, airflow, temperature, moisture/relative humidity, nutrition, competitive microorganisms, etc. These factors influence microbial growth and transmission in the plant.
Food processors are encouraged to aggressively monitor the microbial ecology in their factories with particular reference to Listeria species or Listeria-like organisms on product-contact surfaces and L. monocytogenes in the general (non–product-contact) environment. The bulk of sampling should be taken >3 hr into production. Regular frequencies and locations for sampling should be established but latitude given for taking investigational samples. Understanding where and when Listeria and microbial growth niches (e.g., determined by APC) occur in a processing facility can provide valuable information regarding where and when appropriate interventions should occur.
• Techniques for Sampling the Environment. A number of techniques exist for sampling the factory environment (Evancho et al., 2001). More commonly used approaches include the use of presterilized, inhibitor-free sponges, traditional swabs, and contact plates.
Finished-Product/In-Line Sampling
Product testing is often controversial, given the various assumptions that people make about its efficacy and role in HACCP verification.
• Finished-Product Testing. Finished-product testing cannot be relied on as the sole determinant of a Listeria-free product. No amount of finished-product sampling and testing short of assaying the entire product with a perfect method can guarantee a Listeria-free product.
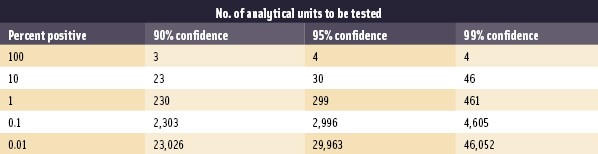 Finding a problem through finished-product testing is likely in situations where the incidence of product contamination is high (Table 2). This is rarely the case in the U.S. Tompkin (2002) stated that "it should be possible in most food processes that include a validated listericidal step (e.g., cooking) to keep the prevalence of product contamination <0.5%." It is impractical to test enough samples to gain high confidence of detecting contaminated lots with such low contamination incidences. Consider a product contaminated at the 1% level. In theory, 299 randomly selected samples per lot are required to gain a 95% chance that at least one sample would test positive (Table 2; Midura and Bryant, 2001). If the true incidence is 0.1%, it would take 2,996 samples per lot, and so forth. Therefore, finished-product testing should be viewed as part of a comprehensive Listeria-control program including GMPs and HACCP and its other prerequisite programs. Knowing where to look, taking appropriate environmental samples and appropriate corrective action, is far more effective than extensive product testing.
Finding a problem through finished-product testing is likely in situations where the incidence of product contamination is high (Table 2). This is rarely the case in the U.S. Tompkin (2002) stated that "it should be possible in most food processes that include a validated listericidal step (e.g., cooking) to keep the prevalence of product contamination <0.5%." It is impractical to test enough samples to gain high confidence of detecting contaminated lots with such low contamination incidences. Consider a product contaminated at the 1% level. In theory, 299 randomly selected samples per lot are required to gain a 95% chance that at least one sample would test positive (Table 2; Midura and Bryant, 2001). If the true incidence is 0.1%, it would take 2,996 samples per lot, and so forth. Therefore, finished-product testing should be viewed as part of a comprehensive Listeria-control program including GMPs and HACCP and its other prerequisite programs. Knowing where to look, taking appropriate environmental samples and appropriate corrective action, is far more effective than extensive product testing.
• In-Line Sampling. Sometimes it is impractical to sample product-contact surfaces of some processing equipment. Rigorous application of statistical sampling techniques at selected locations before and after an inaccessible area has been effective in isolating areas of product contamination.
--- PAGE BREAK ---
Sample Testing and Compositing
Data are only as good as the sampling approach and test method selected.
• Test Methods. Numerous conventional and rapid assays exist for the recovery of Listeria. It is best for companies to use those approved by the appropriate regulatory branch in their particular industry, e.g., Food and Drug Administration (Hitchens, 2003) or USDA (USDA, 2005), and/or by an Official Method of Analysis published by AOAC (Horwitz and Latimer, 2005). Companies should ensure that any methods they use routinely have been scientifically validated for their particular sample matrix.
• Compositing Product Samples. The ability to combine multiple randomly collected samples into one will clearly save testing costs. However, compositing schemes should also be validated. Inappropriate compositing schemes can also lead to misleading results. Curiale (2000) showed that some RTE meat sample compositing schemes yielded inconsistent results, depending on the type of meat product sampled and the Listeria assay performed.
Other approaches to sampling may also afford enhanced recovery of Listeria or reduced labor intensiveness, such as product or package rinses (Wallace et al., 2003).
• Molecular Subtyping. Once the samples are collected and tested, and isolates recovered, a variety of molecular subtyping techniques may be applied, such as PFGE, RAPD, RepPCR, and 16s rDNA sequencing. Manufacturers tend to use specialized laboratories for this work, but some have developed in-house techniques for this purpose. These approaches have been useful in revealing specific patterns of Listeria transmission that would otherwise not have been understood. In-factory Listeria testing is not recommended.
Data Management
Some companies have diligently tracked this microbe and amassed a lot of information. However, important trends can be missed unless one manages these data properly. For example, assume that L. monocytogenes was recovered from a site during post-operational sampling, corrective action is taken, and the site tests negative at the next sampling. The company might assume that corrective action taken at this site was effective. However, analysis of trend data throughout the year may tell a different story. Perhaps the site was positive approximately once per month for 12 consecutive months. Clearly a better corrective action would need to be applied. Eifert and Arritt (2002) has shown how Excel™ Pivot tables can be used for precisely this type of analysis.
References
Buzby, J.C. and Roberts, T. 1996. ERS updates foodborne disease costs for seven pathogens. Food Review, Sept.–Dec., pp. 20-25. www.ers.usda.gov/publications/foodreview/sep1996/sept96e.pdf.
CDC. 2001. Diagnosis and management of foodborne illness: A primer for physicians. Centers for Disease Control and revention. Morbid. Mortal. Wkly. Rept. 50(RR02): 1-69. www.cdc.gov/mmwr/preview/mmwrhtml/rr5002a1.htm#tab1.
CDC. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—Selected sites, United States, 2003. Morbid. Mortal. Wkly. Rept. 53(16): 338-343. www.cdc.gov/mmwr/preview/mmwrhtml/mm5316a2.htm#fig2.
Curiale, M. 2000. Validation and the use of composite sampling for Listeria monocytogenes in ready-to-eat meat and poultry products. Research report prepared for the American Meat Institute Foundation. www.amif.org/pdf/Silliker%20Validation.pdf.
Downes, F.P. and Ito, K. 2001. "Compendium of Methods for the Microbiological Examination of Foods," 4th ed. Am. Public Health Assn., Washington, D.C.
Eifert, J.D. and Arritt, F.M. 2002. Evaluating environmental sampling data and sampling plans. Dairy, Food, Environ. Sanit. 22: 333-339.
Evancho, G.M., Sveum, W.H., Moberg, L.J., and Frank, J.F. 2001. Microbiological monitoring of the food processing environment. Chpt. 3 in Downes and Ito (2001), pp. 25-35.
Faust, R.E., and Gabis, D.A. 1988. Controlling microbial growth in food processing environments. Food Technol. 42(12): 81-82, 89.
Hitchens, A. 2003. Detection and enumeration of Listeria monocytogenes in foods. Chpt. 10 in "Bacteriological Analytical Manual," Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~ebam/bam-10.html.
Horwitz, W. and Latimer, C.W. Jr. 2005. "Official Methods of Analysis," 18th ed. AOAC International, Gaithersburg, Md.
ICMSF. 2002. "Microorganisms in Foods, 7: Microbiological Testing in Food Safety Management." Intl. Commission for the Microbiological Specifications of Foods. Kluwer Academic Plenum Publishers, New York.
Kornacki, J.L. and Johnson, J.L. 2001. Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety Indicators. Chpt. 8 in Downes and Ito (2001), pp. 69-82.
Lou, Y. and Yousef, A.E. 1999. Characteristics of Listeria monocytogenes important to food processors. Chpt. 6 in Marth and Ryser (1999), pp. 131-224.
Marth, E.H. and Ryser, E.T. 1999. "Listeria, Listeriosis, and Food Safety," 2nd ed. Marcel Dekker, New York.
Midura, T.F. and Bryant, R.G. 2001. Sampling plans, sample collection, shipment, and preparation for analysis. Chpt. 2 in Downes and Ito (2001), pp. 13-23.
Tompkin, B.A. 1999. Guidelines to prevent post-processing contamination from Listeria monocytogenes. Dairy, Food, Environ. Sanit. 19: 551-562.
Tompkin, B.A. 2002. Control of Listeria in the food-processing environment. J. Food Protect. 65: 709-723.
USDA. 2003. Control of Listeria monocytogenes in ready-to-eat meat and poultry products; Final rule. Food Safety and Inspection Service, U.S. Dept. of Agriculture, Washington, D.C. Fed. Reg. 68: 34207-34254 (9 CFR Part 430).
USDA. 2005. Isolation and identification of Listeria monocytogenes from red meat, poultry, egg, and environmental samples, Chpt. 8.04 in "Microbiology Laboratory Guidebook." Food Safety and Inspection Service, U.S. Dept. of Agriculture, Washington, D.C. www.fsis.usda.gov/Ophs/Microlab/Mlg_8_04.pdf.
Wallace, F.M., Call, J.E., and Luchansky, J.B. 2003. Validation of the USDA/ARS package rinse method for recovery of Listeria monocytogenes from naturally contaminated, commercially prepared frankfurters. J. Food Protect. 66: 1920-1973.
