Coloring Foods and Beverages
FOOD SAFETY and QUALITY
 The use of color additives derived from natural sources, commonly referred to as natural colors, to color foods and beverages is increasing, and the use of synthetic color additives is decreasing. Major food companies are removing synthetic color additives from their products and replacing them with natural colors to appeal to consumer demand.
The use of color additives derived from natural sources, commonly referred to as natural colors, to color foods and beverages is increasing, and the use of synthetic color additives is decreasing. Major food companies are removing synthetic color additives from their products and replacing them with natural colors to appeal to consumer demand.
The Federal Food, Drug, and Cosmetic Act (FD&C Act) provides that a substance that imparts color is a color additive and is subject to premarket approval requirements unless it is used solely for a purpose other than coloring. The U.S. Food and Drug Administration (FDA) defines a color additive as any dye, pigment, or other substance made or obtained from a vegetable, animal, mineral, or other source capable of coloring a food, drug, cosmetic, or any part of the human body. Regulations regarding color additives are published in Title 21 Parts 70–82 of the Code of Federal Regulations (CFR). The use of an unlisted color additive, the improper use of a listed color additive, or the use of a color additive that does not meet the purity and identity specifications of the listing regulation may cause a product to be adulterated and subject to FDA enforcement action.
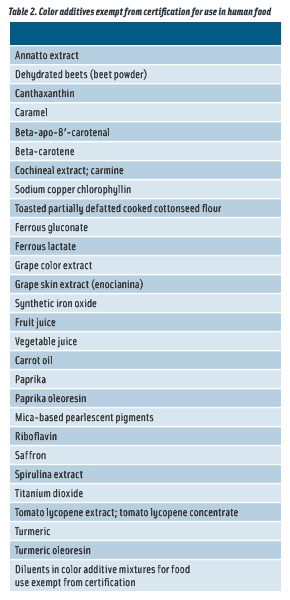
 The FDA classifies color additives as either certified color additives or color additives exempt from certification. The color additives subject to certification for use in food, principally the FD&C colors, must be certified by the FDA as meeting the identity and purity specifications and use limitations described in 21 CFR part 74 (see Table 1). Color additives exempt from certification are generally produced from plant, animal, and mineral sources; they do not require certification but must meet the identity and specifications described in 21 CFR part 73 (see Table 2).
The FDA classifies color additives as either certified color additives or color additives exempt from certification. The color additives subject to certification for use in food, principally the FD&C colors, must be certified by the FDA as meeting the identity and purity specifications and use limitations described in 21 CFR part 74 (see Table 1). Color additives exempt from certification are generally produced from plant, animal, and mineral sources; they do not require certification but must meet the identity and specifications described in 21 CFR part 73 (see Table 2).
• Petitioning for new colors or new uses. Any interested person may petition the FDA to list a new color additive or a new use of a listed color additive. The petitioner must provide information on the identity of the proposed color additive; its physical, chemical, and biological properties; chemical specifications; manufacturing process; stability data; intended uses and restrictions; labeling; tolerances and limitations; analytical methods for enforcing chemical specifications; analytical methods for determination of the color additive in products; identification and determination of any substance formed in or on products because of the use of the color additive; safety studies (including toxicology data); estimate of probable exposure; proposed regulation; environmental assessment; and a reason why certification is not necessary if exemption from batch certification is requested. If the petition is accepted for filing, the FDA will publish a notice of the petition’s filing in the Federal Register, then evaluate the data in the petition, review the comments and other relevant data in its files, and if appropriate, issue a final regulation for the color additive, which will become effective 31 days after publication, provided that no objections are received. This process may take months for a straightforward extension to years for a novel color source.
• Certifying batches. A sample of each newly manufactured batch of a certifiable color additive must be submitted to the FDA to ensure that it meets the identity and specification requirements of its listing regulation. This certification process is described in 21 CFR part 80. The company requesting certification must submit to the FDA a four-ounce sample from each batch along with the name of the color additive, the name of the manufacturer, storage conditions, the use for which it is being certified, and the batch weight, on which the FDA bases its fee for certification. Prior to certification, the batch cannot be used and must be stored separately from batches already certified.
Upon receipt of the sample, the FDA analyzes it for appearance; purity (total color content); moisture; residual salts; unreacted intermediates; colored impurities (subsidiary colors); other specified impurities; and the heavy metals lead, arsenic, and mercury. If the sample meets the requirements in the listing regulation for the color additive, the FDA issues a certificate for the batch that identifies the color additive, the batch weight, the uses for which the color additive is certified, the name and address of the owner, and other required information. It also assigns a unique lot number to the batch and changes the name of the batch from, for example, “tartrazine” to “FD&C Yellow No. 5.” The agency may inspect the manufacturer’s establishment, examine records of use of certified color additives, and take samples from certified batches for analysis for comparison with the FDA’s original results.
• Labeling. All color additives, whether certified or exempt, are considered artificial for labeling purposes in the United States, and the addition of color to a product must be identified on the label. Labeling of any food containing color additives that the FDA certifies for food use, such as FD&C Red No. 40 and FD&C Yellow No. 5, must declare those additives by name. Color additives exempt from certification may be labeled in a variety of ways, such as “Colored with _______,” “Color added,” “Contains artificial color,” “Artificial color added,” or similar statements.
Use of the term “natural color” is not permitted as the FDA considers all color additives to be artificial. The agency has long considered natural to mean that nothing artificial or synthetic, including colors regardless of source, has been included in or added to a product that would not normally be expected to be present. On November 12, 2015, in response to several citizen petitions, the FDA published in the Federal Register a request for information and public comments regarding whether it is appropriate to define natural, how the agency should define it, how the agency should determine its appropriate use on food labels, and other questions. The deadline for comments is May 10, 2016.
--- PAGE BREAK ---
White Paper Proposes Standards for Natural Colors
A white paper titled “Establishing Standards on Colors from Natural Sources (Exempt From FDA Certification): An Expert Committee Report” will soon appear in the journal Concise Reviews and Hypotheses in Food Science, published by the Institute of Food Technologists (IFT). During the IFT15 show, James Simon, director of the New Use Agriculture and Natural Plant Products Program at Rutgers University, said that the white paper is intended to initiate discussion among academic, research, industry, and regulatory communities to provide a realistic, practical approach to refine existing standards for colors from natural sources. Whereas the certification process for FD&C lakes and dyes includes a high level of quality control and safety evaluation, he said, there are few product definitions or publicly available purity, quality, and safety specifications for colors from natural sources, and the specifications that do exist are not consistently applied. The listing regulations for colors exempt from certification provide identity and purity specifications and use limitations and establish some basic standards for these substances, he said, but more is needed. The white paper outlines a set of standards that could be the framework for the industry.
Some of the major points of the white paper are that natural colorants must be properly authenticated to verify that the material is what it is claimed to be. Since colors from natural sources are crop-based, the potential exists for microbial contamination, so they should be tested for spoilage organisms, indicator organisms, and foodborne pathogens. There is also a potential for pesticide residues to be present and concentrated during the extraction process as well as a risk of adulteration, such as by toxic substances or dyes. Since organic solvents are used extensively for the extraction, purification, and concentration of natural colorants, removal of the solvents is extremely important because of risks associated with exposure to them. Analytical methods must be robust, reliable, and accurate but must also allow for a realistic approach that the industry will accept, permitting greater monitoring and implementation and creating random checks along the value chain.
In addition, there is a lack of harmonization among regulatory bodies: Some manufacturers prefer a loosely regulated or unregulated field because of concerns that innovation and the ability to form strategic partnerships will be limited. It may be difficult to reach agreement on standards or methods of identification and analysis since natural sources are complex mixtures of chemical compounds that vary considerably due to environmental, handling, and processing factors. The white paper recommends that the industry begin drafting its own regulatory framework and standards so that it is not done for the industry in a way that is less agreeable. Essentially, food manufacturers who use colors from natural sources should require their suppliers to test according to the standards proposed in the white paper to ensure brand protection and safety for their consumers.
During the IFT15 symposium “Overview of Natural Colors: Sources and Chemistry,” Monica Giusti, associate professor, Dept. of Food Science and Technology, Ohio State University, said that colors are used in food to enhance and correct colors already present, provide color identity to colorless foods, and account for color loss during storage. She described the main classes of pigments in plants and the colors they provide, including chlorophylls (blue-green), carotenoids (yellow to orange to intense red), anthocyanins (orange to red to purple to blue), and betalains (yellow to purple-red). She reviewed the legislative history of food colors in the United States and described the main classes of pigments in plants and the colors exempt from certification. She said that the challenges of transitioning from synthetic color additives to color additives from natural sources include finding the right color, ensuring compatibility with the matrix, providing color and pigment stability, and eliminating possible undesirable aromas/flavors. The advantages, she said, are favorable consumer perception, increased demand, standardization of formulations, and potential health benefits.
In his IFT15 presentation “Natural Color Technology: Processing and Applications in Food Systems,” Steve Talcott, professor of food chemistry, Texas A&M University, described natural color sources and the processes and solvents used in their production. He described the considerations in choosing a natural color, including supply chain lead time and storage conditions, product appearance (color intensity, clarity), product properties (pH and matrix composition), interactions and order of addition, processing (enzymes, oxidizers or interactions, time, and temperature), storage (warehouse, distribution, and retail), and consumer handling.
He described natural colors that could replace certified colors, such as turmeric and beta-carotene for Yellow No. 5; annatto, beta-carotene, apo-carotenoids, and paprika for Yellow No. 6; beets, anthocyanins, and carmine for Red No. 40; anthocyanins/beet, huito, and spirulina for Red No. 40/Blue No. 1; huito and spirulina for Blue No. 1; and turmeric, beta-carotene, huito, and spirulina for Blue No. 1/Yellow No. 5. He discussed anthocyanins in detail, saying that they can be sourced from most edible fruits and vegetables, are most stable in high-acid environments, provide acceptable light and heat stability, and can be enhanced with added co-pigments.
 Symposium Addresses Color Additive Safety & Regulation
Symposium Addresses Color Additive Safety & Regulation
During the IFT15 symposium “The Science Behind the Safety: Exploring Global Challenges to Natural and Synthetic Colors,” Sarah Codrea, executive director, International Association of Color Manufacturers (IACM), said that despite thorough reviews by scientists and regulators and decades of safe use, public interest groups and media outlets regularly question the safety of food colors. She described the activities of the IACM, which represents the natural and synthetic color additives industry and the color user community. The organization provides scientific and regulatory expertise and advocates global harmonization of standards and regulations.
George Pugh, director of ingredient safety, Coca-Cola Co., discussed myths and rumors about synthetic colors, specifically that synthetic dyes cause hyperactivity in children. He discussed the University of Southampton study that suggested that some mixtures of certain artificial food colors and benzoate preservative may affect the level of hyperactive behavior in children. He pointed out the following problems with the study: behavior assessment data were not collected for the respective placebo phases, the observed effects lacked clear statistical significance, behavioral changes were only partially significant, the very weakly statistically significant effects were only measured under a constant seven-day treatment period, and no biological mechanism for causal association between the intake of the corresponding additives and the onset of hyperactivity could be derived from the results.
He said that the European Food Safety Authority (EFSA) concluded that the significance of the effects on the behavior of the children was unclear since it was not known if the small changes in attention and activity observed would interfere with schoolwork or other intellectual functioning. The EFSA noted that the study was unable to pinpoint which additives may have been responsible for the effects observed in the children since mixtures and not individual additives were tested.
David R. Schoneker, director of global regulatory affairs, Colorcon, reviewed the FDA’s legal framework for regulation of color additives. He reviewed the status of draft and adopted provisions for colors in the Codex Alimentarius Commission’s General Standard for Food Additives and the EFSA and the Joint FAO/WHO Expert Committee on Food Additives evaluations of the safety of certain colors. He also described how the European Union (EU) and the United States regulate color additives. In Europe, he said, all food additives are given labeling codes commonly referred to as E-numbers, (e.g., allura red is labeled as E129 and amaranth as E123). He mentioned that the EU also requires a warning label for the Southampton colors, saying that they may have effects on activity and attention in children. An FDA advisory committee, however, after review of all scientific data, concluded that there was no causal relationship and a warning label is not needed. He said that the IACM does not support a warning for colors on ingredient labels because there is no proven causality to hyperactivity and, as with all food ingredients, if consumers want to avoid a specific ingredient, they can see it listed on the label and make an informed product choice.
--- PAGE BREAK ---
Q&A with Sensient Food Colors
Mark Goldschmidt, director of global quality control and product safety, Sensient Food Colors LLC, St. Louis, Mo. (sensientfoodcolors.com), answered questions about natural and synthetic color additives.
Q: How large is a batch of certified color?
Goldschmidt: Once a batch is certified, it becomes an FDA lot by definition. Since there are no requirements on the size of a batch requiring certification, it can vary greatly from a small batch to several tons of product.
Q: What tests do you conduct on each batch of certified color?
Goldschmidt: We run at a minimum all the tests listed under the appropriate color regulation in 21 CFR part 74. These are the same tests the FDA runs to determine if the color complies with the regulation and can be certified.
Q: What tests do you conduct on each batch of natural color?
Goldschmidt: We run all the tests listed under the appropriate color regulation in 21 CFR Part 73. However, there are very few specifications listed in these regulations. This is the whole purpose of a white paper [“Establishing Standards on Colors from Natural Sources (Exempt From FDA Certification): An Expert Committee Report”] prepared by an expert committee convened by Sensient and the U.S. Pharmacopeial Convention (USP) that focuses on establishing safety standards for colors from natural sources.
Q: Are any microbiological tests done?
Goldschmidt: We conduct comprehensive microbiological testing on both natural and certified colors. We also test for heavy metals, pesticide residues, residual solvent, and other adulterants. This is currently not an industry standard but something we hope will become such in the future. With the forthcoming publication of the white paper, we expect further discussion about the issue and its importance.
Q: Once a batch is certified by the FDA, what do you do?
Goldschmidt: The batch is labeled with an FDA lot number and then released for sale or used to make other color products. All documents (certificates of analysis, labels, etc.) must list the FDA lot number.
Q: Can the batch be used to provide color to more than one food company?
Goldschmidt: Yes. It can also be used by the color manufacturer in downstream color-manufacturing processes.
Q: What tests does a food company do on the certified color it receives?
Goldschmidt: This varies from company to company. Quite frequently, no additional testing is done on certified colors. When additional testing is undertaken, those tests listed in the Food Chemicals Codex would be the ones most packaged-food companies would employ.
Q: What instruments do you use to conduct the tests?
Goldschmidt: Typical instrumental methods used in testing colors include ultraviolet/visible spectrophotometry for identification, inductively coupled plasma for heavy metals, thin-layer chromatography or high-performance liquid chromatography and liquid chromatography–mass spectrometry for impurities or adulteration.
Q: There are many companies supplying colors to the food industry. How do their products differ?
Goldschmidt: Products can differ substantially. Some color providers focus on single-source expressed juices that might be appropriate only as building blocks to achieve desired shade targets. Other suppliers, Sensient included, focus on color solutions designed to address stability challenges and hit specified shade targets for different applications. The appropriate natural color source will vary considerably depending on the application.
Q: Have there been any major advances regarding certified and natural colors?
Goldschmidt: There have been quite a lot of developments in natural colors. Novel color sources such as spirulina have been approved for use in certain applications. Additionally, technological advancements such as new purification technologies have closed the performance gap between certified and natural colors. Additionally, natural plant-breeding techniques are narrowing the cost-in-use gap between natural and certified colors. Most of the innovation has been focused on the natural side since that is where the market seems to be headed.
Q: What are the major challenges ahead regarding certified colors?
Goldschmidt: The challenge will probably be to counter the public perception that they could have an adverse effect on human health. The reality is that we have quite a lot of data that don’t support that claim, but the studies that do support such claims tend to get more attention.
Q: What are the challenges ahead regarding natural colors?
Goldschmidt: The industry needs to develop and agree on standards for natural colors, ideally a self-regulated set of standards. The risk to the industry is that if we were to have even one food safety incident involving natural colors, it could negatively impact the perception of natural color and inevitably food color overall.
 Neil H. Mermelstein, IFT Fellow,
Neil H. Mermelstein, IFT Fellow,
Editor Emeritus of Food Technology
[email protected]


