Setting Standards for Cannabis Edibles
FOOD SAFETY AND QUALITY
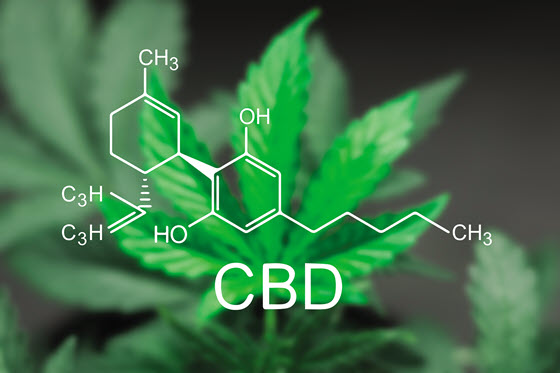
The cannabis plant (Cannabis sativa) contains more than 80 biologically active compounds, including delta-9-tetrahydrocannabinol (THC), which causes psychoactive effects, and cannabidiol (CBD), which does not. Cannabis containing high levels of THC is referred to as marijuana and is regulated as a drug. Cannabis containing no more than 0.3% THC on a dry weight basis is referred to as hemp and was removed from regulation as a drug by the 2018 Farm Bill. Use of marijuana in the United States is considered illegal by the federal government, but 19 states and the District of Columbia (DC) have legalized it for medical use, and 11 states and DC have legalized it for recreational use. Hemp, and therefore CBD, is federally legal in all 50 states and DC and has been used by some companies in food and beverage products not marketed in interstate commerce.
Only three cannabis-related products have been approved by the U.S. Food and Drug Administration (FDA): Marinol and Syndros, which contain a synthetic THC for treatment of anorexia and AIDS, and Cesamet, which contains a synthetic compound similar to THC for treatment of severe nausea and vomiting caused by cancer drugs. Many people and companies have become interested in CBD for its purported but unproven benefits for treating a wide variety of health issues (primarily for managing anxiety, insomnia, and chronic pain), but only one cannabis-related drug has been approved by the FDA: Epidiolex, a purified form of CBD, for treatment of seizures in children.
The FDA published in the April 3, 2019, Federal Register an announcement of a public hearing and a request for scientific data and information about products containing cannabis or cannabis-derived compounds, saying that there are many unanswered questions about their safety. The agency raised numerous issues, such as what the functional purpose is for adding cannabis-derived compounds such as CBD to foods and what evidence is avail-able to demonstrate that it has the intended or perceived effect, whether there are standards or processes to ensure manufacturing quality and consistency of products, whether validated analytical testing is needed to support the manufacturing of safe and consistent products, and whether making CBD widely available in foods or dietary supplements might reduce the incentives to conduct clinical research on it.
A public hearing took place on May 31, 2019; more than 600 people attended in person and more than 2,300 joined remotely, and more than 100 speakers gave presentations. Nearly 4,500 comments were received by the time the public docket closed on July 16, 2019. The FDA formed an internal policy working group to review the comments and consider what statutory or regulatory changes might be needed for dietary supplements and conventional foods containing CBD to be lawfully marketed as well as what the impact on public health would be. The working group was expected to report on its progress by early fall.
Organizations Address Standards
Regardless of the CBD-infused food or beverage a company wants to make, the product must meet state regulatory requirements and have an accurate analysis of the CBD content. Analytical laboratories, both industrial and regulatory, and instrument companies have developed methods for analyzing products for THC and CBD con-tent, heavy metals, microbial and chemical contaminants, and other analytes, but there is no standardization or harmonization of these methods yet. Various organizations are working to set analytical standards and address other cannabis issues.
∙AOAC International. The standards-setting organization AOAC International established the Cannabis Analytical Science Program (CASP) in 2019 to approve consensus methods for the analysis of cannabis, hemp, and related food products. Among the program’s objectives are to facilitate the development and publication of cannabis and hemp-specific methods and standards; identify cannabis and hemp reference materials; establish a cannabis proficiency testing program; provide analytical and laboratory management training, particularly ISO accreditation training; and provide information regarding cannabis and hemp to regulators and legislators. The ultimate goal is to harmonize analytical methods and make them as uniform as possible to create a common set of standards, setting limits of quantification that will be accepted internationally and that will be accurate and repeatable for the entire cannabis industry, not just state by state.
In March 2019, CASP formed three working groups: Cannabinoids in Consumables, Chemical Contaminants in Cannabis, and Microbial Contaminants in Cannabis. The groups have drafted Standard Method Performance Requirements (SMPRs) that were approved during the September 2019 AOAC Annual Meeting & Exposition. The SMPRs will be used to evaluate laboratory methods submitted for approval as AOAC Performance Tested Methods (PTMs) and Official Methods of Analysis (OMAs).
CASP plans to develop cannabis-related SMPRs and OMAs for potency, pesticide residues, biological contaminants, chemical contaminants, and mycotoxins; untargeted testing profiles; and method validation guidelines. The SMPRs are for cannabinoids in cannabis concentrates, dried plant materials, and chocolate and for pesticides in cannabis. The OMAs are “Cannabinoid in Dried Flowers and Oil Liquid Chromatographic Method” and “Quantitation of Cannabinoids in Cannabis Dried Plant Materials, Concentrates, and Oils Using Liquid Chromatography–Diode Array Detection Technique With Optional Mass Spectrometric Detection.” CASP is also actively engaged in proficiency testing and training programs.
The companies supporting CASP include ABC Testing, Association of Food & Drug Officials (AFDO), Bia Diagnostics, Bio-Rad, Industrial Laboratories, Materia Medica Labs, MilliporeSigma, Pathogen Dx, PerkinElmer, R-Biopharm AG, SCIEX, Supra R&D, TEQ Analytical, Titan Analytical, and Trilogy Analytical. Partners include CEM Corp., CV Sciences, Eurofins Scientific, and Trace Analytics. Affiliates include Charm Sciences, Crystal Diagnostics, Hygiena, Institute of Food Technologists, Lazarus Naturals, Medicinal Genomics, and SC Labs.
Scott Coates, CASP program leader and senior director, AOAC Research Institute, said that training and education of analysts is a major challenge to this brand-new industry that is not used to operating under Good Manufacturing Practices (GMPs) common in the food industry. Third-party validated methods such as PTMs and OMAs, he said, form the basis for implementing GMPs in this new industry. AOAC International points out that it does not advocate for or against the use or legalization of canna-bis and will not accept funding from any organization involved in the cultivation, manufacture, distribution, or possession of cannabis as long as it is illegal in the United States.
∙ ASTM International. The standards setting organization ASTM International in 2017 formed the International Technical Committee D37 on Cannabis to develop voluntary consensus standards for cannabis and its products and pro-cesses, focusing on quality and safety. The committee has eight technical sub-committees: Indoor and Outdoor Horticulture and Agriculture; Quality Management Systems; Processing and Handling; Security and Transportation; Personnel Training, Assessment, Credentialing; Industrial Hemp; Cannabis Devices and Appliances; and Laboratory.
The laboratory subcommittee is working on numerous projects, such as a guide for sensory and consumer evaluation of products containing THC or CBD for recreational or medicinal use; mini-mum requirements for quality assurance and quality control for cannabis analytical methods and laboratories; best laboratory practices, recommended certifications, and recommended types of analyses typically required in the cannabis industry; and a guide describing the minimum requirements for stability testing of new cannabis and cannabis-based products for determining appropriate storage conditions and shelf life.
The laboratory subcommittee is also working on specific analytical techniques, including analyses of terpenes in cannabis using gas chromatography–tandem mass spectrometry, analyses of trace elements in cannabis by inductively coupled plasma–mass spectrometry, analysis of residual solvents in cannabis oil, and determination of cannabinoids in thermally prepared food products using thermal desorption gas chromatography/mass spectrometry or gas chromatography flame ionization detection. Jeremy Melanson, organic chemical metrology team leader at Canada’s National Research Council and chair of the ASTM laboratory subcommittee, said that the wide range of potential products will require a suite of different methods that may need to be validated for each sample type.
∙ American Chemical Society. The American Chemical Society (ACS) established a Cannabis Chemistry subdivision (CANN) within its Chemical Health & Safety division in 2014 with the vision of being recognized as the leading chem-istry authority on the processing, extraction, and analysis of cannabis products. Some of CANN’s members are engaged in efforts to develop better analytical methods for cannabis products, focusing on sample preparation of edible products with complex matrices.
Melissa Wilcox, global sales and market development manager at Regis Technologies and secretary of CANN, said that the challenges for manufacturers formulating and producing food products with CBD and other cannabinoids are mainly consistency in achieving an accurate concentration of cannabinoid in their product; stability of the cannabinoids in a specific matrix; and accurate analysis/measurement of cannabinoid concentration, especially in difficult matrices such as gummies, caramels, taffy, chocolate, and baked goods. Each new type of CBD-infused product becomes a method development project, she said, and testing labs don’t always have the resources and time to devote to frequent method development.
Wilcox’s collaborative research team, which included James Neal-Kababick, director of Flora Research Laboratories, developed a method that called for grinding the sample using liquid nitrogen and Celite (a fluffy silica) and doing essentially an on-line extraction using flash chromatography, which at the same time allowed for the separation (and collection) of the cannabinoid fractions from the fat, sugar, and other additives in the sample. The cannabinoid fractions were then analyzed by high-performance liquid chromatography without the fear of interferences from the sample matrix. Another CANN member, David Dawson, research principal at CW Analytical Laboratories, reported at the ACS national meeting in August 2019 that his work on analysis of THC content in chocolates indicated that components in chocolate might be interfering with can-nabis potency testing, leading to inaccurate results. He plans to extend his work to analysis of CBD and to other edibles such as chocolate chip cookies.
∙ American Herbal Products Association. In March 2019, the American Herbal Products Association (AHPA) issued a guidance policy on dietary supplements and foods containing hemp and hemp-derived CBD to help ensure that, among other things, the industry complies with applicable federal regulations, including food facility registration; current good manufacturing and good agricultural practice regulations; and labeling requirements.
Holly E. Johnson, chief science officer at AHPA and chair of the AOAC Cannabinoids in Consumables working group, said that AHPA created a Cannabis Committee in 2010 and has released a series of guidance documents related to hemp and cannabis cultivation, processing, manufacturing, dispensing, and laboratory practice. The laboratory best practice rules complement existing good laboratory practices and focus on personnel, security, sample handling and disposal, and data management and reporting activities that may be unique to laboratories analyzing cannabis samples. They were offered in the form of a draft regulation for possible adoption by state and local regulatory authorities. Johnson added that the pathway for use of CBD as a food additive is not clear. In July 2019, the AHPA urged the FDA to act promptly to protect consumers by issuing a regula-tion permitting hemp-derived CBD as a lawful ingredient in foods and dietary supplements.
∙ Institute of Food Technologists. In 2017, IFT members formed a Legalized Cannabis and Hemp Edibles interest group on the IFT Connect online platform for members to share information and discuss the safety, quality, formulation, regulatory, and policy challenges of cannabis edibles.
Food Technology Articles

Battling Biofilms
In this column, the author describes the stages of biofilm development in food processing plants, methods of removal, and best practices for prevention.
Ensuring Quality and Safety in Fried Foods
In this column, the author describes basic principles for maintaining frying oil quality and safety.
Getting a (Drying) Bead on Crop Preservation
Drying Beads offer an effective solution to drying crops in moist environments and can be a valuable resource in developing countries.
How to Fast Track Your Shelf Life Testing
In this column, the author describes considerations and watchouts when using accelerated shelf-life testing (ASLT), which has emerged as a technique that simulates conventional shelf-life testing in a fraction of the time.
Making a Successful Co-Manufacturing Match
Finding the right co-manufacturing partner can be challenging, but it can also be a smart business strategy. Here’s a look at some of the ins, the outs, and the how-tos.