Automated Assay Maps Persistent Listeria
FOOD SAFETY AND QUALITY
Listeriosis is a serious infection caused by consumption of foods contaminated by Listeria monocytogenes. According to the U.S. Centers for Disease Control and Prevention (CDC), listeriosis is responsible for 1,600 cases of foodborne illness and about 260 deaths in the United States each year and is the third leading cause of death from foodborne illness.
Rheonix and Mérieux NutriSciences announced in July 2019 the results of a case study designed to show how Rheonix’s Listeria PatternAlert Assay in combination with Mérieux’s EnviroMap visualization software can help food processors identify where Listeria may be persisting in their facilities and take prompt corrective action. Based on the success of its early-access customer evaluations, including the three-month trial with Mérieux NutriSciences, Rheonix began marketing the assay directly to food companies and commercial testing laboratories in September 2019. Mérieux NutriSciences began offering the assay to its clients in fall 2019 and plans to add it to the services it provides in more than 100 testing laboratories around the world.
A Solution to Ineffective Screening
Food producers routinely monitor their processing and packaging facilities for the presence of Listeria as an indicator that the pathogenic species L. monocytogenes could be present and take corrective action when Listeria is found. The U.S. Food and Drug Administration (FDA) collects and tests environmental samples from food facilities that produce what are considered high-risk products, and when samples are found positive for L. monocytogenes, the agency characterizes the strains through whole genome sequencing. Samples are subjected to culture-based methods, and isolates are sequenced. If sequenced strains are found to be associated with human illness and are epidemiologically linked to a product manufactured in that facility, the manufacturer could face significant regulatory action and liability. In addition, in cases where significant rates of positive samples are found during initial screening, the FDA can sample again; if the agency finds the same strain again, it can conclude that the strain is persisting in the production environment. A persistent strain can represent a significant food safety risk if it has the ability to contaminate ongoing production and can be an indication that good manufacturing practices, such as sanitation, may not be adequate.
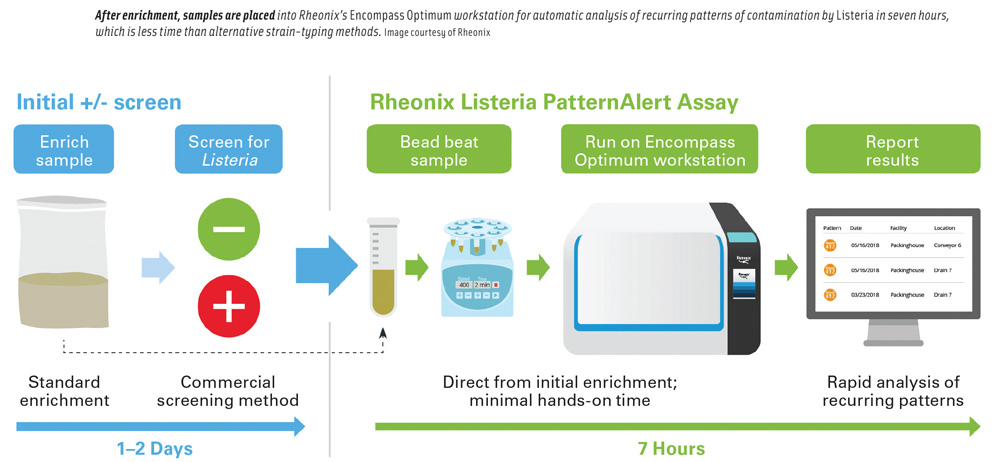
Brooke Schwartz, vice president of strategy and marketing at Rheonix, said that food producers need a way to quickly determine whether Listeria found in their facilities is persistent so that they can take rapid corrective action. The routine use of strain-typing methods, including whole genome sequencing, has not been practical for facilities to implement because of the cost and time involved. Alternative strain-typing methods require an isolate in pure culture and take four to eight days to complete after a presumptive positive screening result. She said that Rheonix designed its Listeria PatternAlert Assay to overcome these drawbacks. The assay is run on Rheonix’s fully automated Encompass Optimum workstation and provides results in as few as seven hours after a positive screening result for less than half the cost of alternative strain-typing methods. She said that the assay can be used for finding recurrent Listeria contamination spots and harborage sites in a facility, identifying the spread of Listeria within a facility, and tracing it back to its source. It can also identify a common pattern across multiple facilities in the same time period, which can provide an early alert to potential contamination introduced by a supplier or other external source.
How the Assay Works
The Listeria PatternAlert Assay is designed to follow a user’s routine environmental Listeria screening workflow. Morgan Wallace, scientific director for applied markets at Rheonix, said that the fact that the assay works directly from an enriched sample enables it to fit into a company’s routine screening workflow. He added that the company’s bioinformatics team identified genetic regions that were not present in any non-Listeria genera and were variably present in Listeria strains. This allows the characterization of Listeria even in the presence of an excess of other bacteria, enabling direct use of the method on enrichments without the need to isolate Listeria in pure culture.
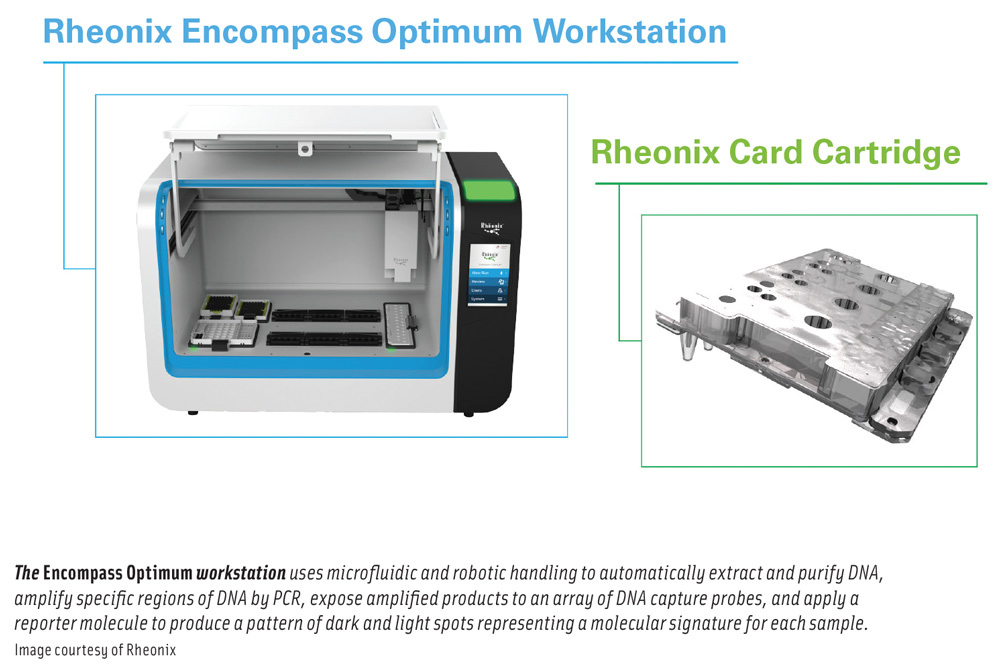
Once a standard screening method indicates that a sample is presumptively positive for Listeria species or L. monocytogenes, an aliquot of the original enrichment is subjected to a short bead-beating step to disrupt the bacterial cells, then loaded onto the Encompass Optimum workstation along with prepackaged reagents and consumables. The fully automated workstation can run up to 24 samples at a time. Fifteen targets that are variably present in Listeria strains are amplified and detected as a pattern of plus/minus reactions on a detection array.
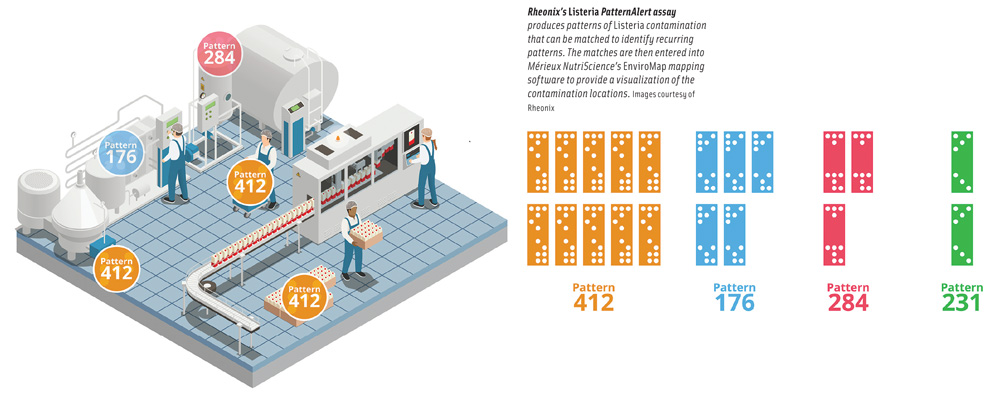
Using microfluidic and robotic handling, the workstation automatically conducts DNA extraction and purification, followed by amplification of specific regions of DNA within Listeria genomes by polymerase chain reaction, exposure of amplified products to a low-density array of DNA capture probes, and application of a reporter molecule. Specific targets that are amplified will bind to complementary probes on the microarray, and when the bound targets are exposed to a reporter molecule, the capture-probe spots will darken; capture-probe spots for which no targets were amplified remain unchanged (light). The resulting pattern of dark and light spots represents a molecular signature for each sample and can be uploaded onto the user’s company-specific database and compared with all other patterns the user has encountered. A report is generated, identifying common patterns across locations and time. This allows for tracking of recurring patterns and detection of new ones, Wallace said.
Schwartz said that Rheonix technology and the Encompass family of workstations have broad application in multiple life-science markets and have been used in other applications. The company has commercialized the Beer SpoilerAlert assay, which is capable of detecting more than 60 different bacteria, yeast, and hop resistance genes from a single sample, and is working on additional assays for other food and beverage testing. Rheonix also recently launched the NGS OnePrep solution, which automates nucleic acid extraction and library preparation for next-generation sequencing applications and is concluding clinical trials in support of a submission to the FDA for 510(k) clearance for a multiplexed sexually transmitted infection assay. In addition, the company is collaborating with the Canadian Food Inspection Agency on typing and detection of high-consequence viral diseases of poultry and swine. Schwartz added that the company has also shown proof of concept for a Zika dual assay and an HIV dual assay for research use, each of which simultaneously detects both host antibodies and viral RNA.
The Case Study
Rheonix partnered with multiple early-access customers (including food testing laboratories, food companies, and food industry associations) to evaluate the assay. Collectively, they conducted thousands of tests on real-world samples. In the most comprehensive evaluation, Mérieux NutriSciences collected and analyzed Listeria environmental sample enrichments from participating clients and used the Listeria PatternAlert Assay to identify different Listeria patterns specific to each facilities’ environment and to understand the frequency of sample patterns, determine root causes, and consider alternative sanitation measures.
The case study detected four different Listeria patterns at various prevalence levels within one of the manufacturing facilities studied. One observed pattern was persistent with 67% of positive Listeria events. It was observed 14 times on the floor near the dock door in the warehouse and 20 times near the floor by the warehouse freezers and was found to be spreading toward one of the production lines. Timothy Freier, vice president of scientific affairs and microbiology at Mérieux NutriSciences North America, said that using mapping software to plot the prevalence of the persistent pattern in the facility showed that the source of the pattern was the warehouse, and the company took actions to reduce the occurrence of the persistent pattern, including conducting an in-depth review of the warehouse floors for visible cracks, wearing, and unsealed areas around dock doors and freezers; evaluating the flow into the freezers by production and warehouse workers to prevent cross-contamination; separating staff from high- and low-risk areas to prevent the spread of bacteria; and installing sanitizing footbath mats and foamers at entry points to the production area.
Having been involved in solving environmental pathogen contamination issues for more than 25 years, Freier said that hunting an invisible foe in the complex food manufacturing environment can be a difficult and frustrating endeavor. He said that combining the Listeria PatternAlert Assay with Mérieux NutriScience’s EnviroMap environmental mapping software to visualize exactly where in the facility the contamination is found will be a big step forward for the food industry. Rather than taking a variety of corrective actions and hoping the problem goes away, which he calls the “spray and pray” method, food manufacturers can take a very targeted and efficient approach to tracing the root cause of contamination events and implement corrective actions that will ensure that the problem does not recur. In the case study, the analytical results were available at roughly half the cost and time of traditional subtyping methods and produced fast, actionable data to select and verify the most effective corrective actions.
Freier said that the two systems—the Rheonix workstation and the Mérieux NutriSciences mapping software—are separate at present. The Listeria PatternAlert Assay tells whether the strain has been seen before or is new to the facility, and the EnviroMap software shows on a map of the facility where the contamination occurred and when. Freier said that building a database of this information enables the user to page back through time, perhaps on a weekly basis over a six-month period, and track how individual strains enter and move around the facility. With such a historical database, he said, manufacturers can move from being reactive to more predictive and proactive so that if they have contamination issues in the future, they can move more quickly to solve them and avoid major problems and potential recalls. In the test case, which was conducted as an example of how the data can be put to practical use, the assay results were manually entered into the mapping software, but teams from both companies are working together to combine them so that the assay results automatically provide a map of contamination in the facility. He said that they hope to have the combination available early next year.