Controlling Moisture in Foods Using Packaging
PACKAGING
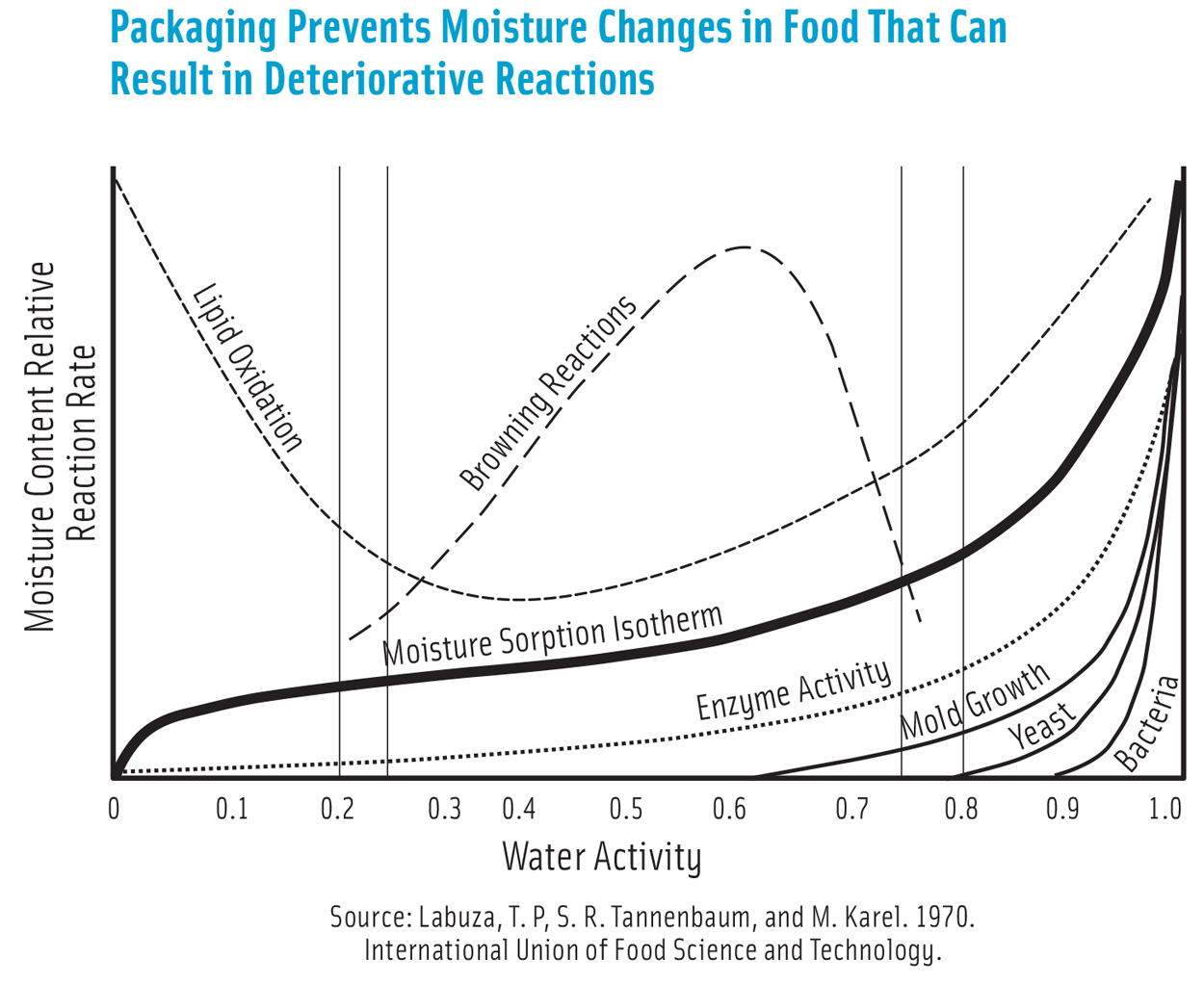
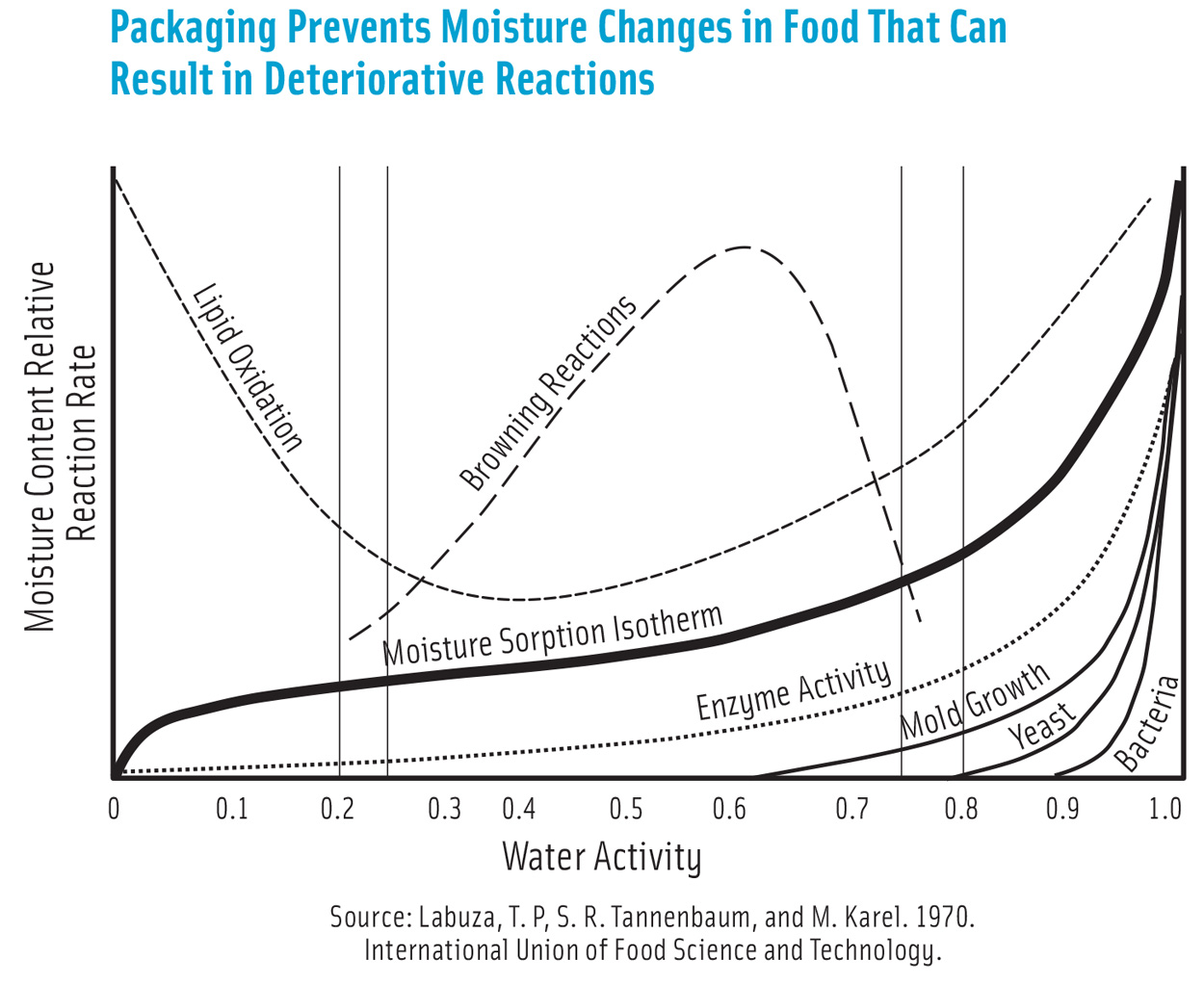
Moisture content affects food texture, nutrient, and flavor profiles and impacts the quality and safety of food. The reaction rates of lipid oxidation, microbial growth, and browning are altered when food moisture content changes. Moisture loss can result in economic loss too, in foods such as produce that are sold by weight.
Effective packaging can play a major role in maintaining product moisture to extend the shelf life of food. Moisture loss and gain from packaged food occurs due to poor seals, pinholes, and punctures, as well as from water vapor permeation through the actual package material itself. Selecting packaging materials with sufficient puncture, tear, burst, and tensile strength can help prevent moisture loss and gain during distribution and handling.
Water Vapor Permeability
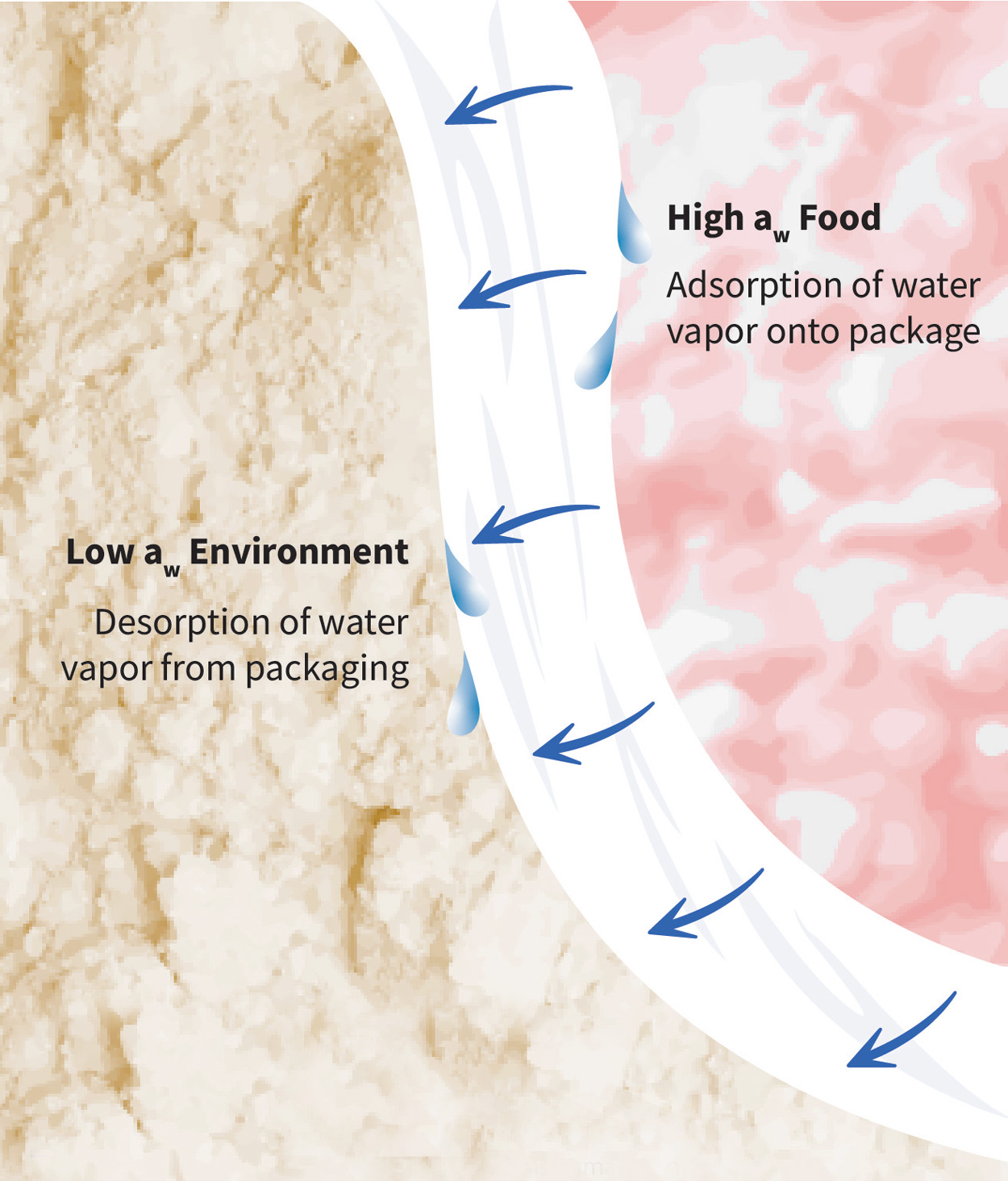
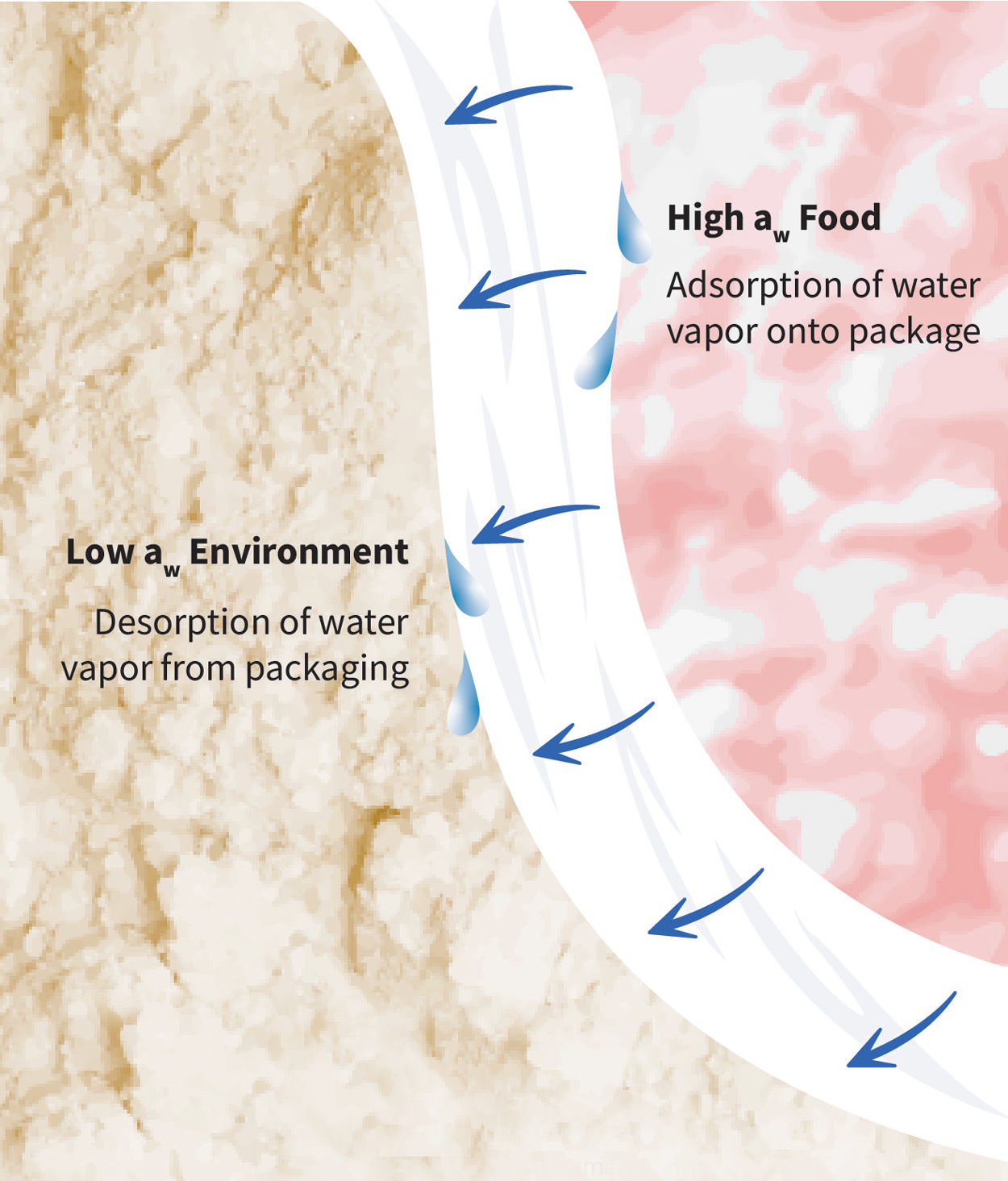
The water vapor permeability (WVP) of a package describes the rate at which water vapor passes through a package when it is exposed to a given water activity or relative humidity (RH) gradient. Water vapor permeation is a function of the solubility and diffusion of water vapor in a package material. It is divided into three steps: 1) water vapor adsorption onto the package, 2) water vapor diffusion through the package, and 3) water vapor desorption from the package. The driving force is from high to low water activity or RH.
The first and third steps of water vapor permeation are concerned with the solubility of water vapor in the package structure. Henry’s law of solubility conveys the relationship between the concentration of permeant within the package material and the permeate in the adjacent air—which can be the package headspace or the air external to the package. A low solubility to water vapor means the package material does not readily adsorb or desorb water vapor. When solubility is low, less water vapor is available for subsequent diffusion through the package material.
The second step of water vapor permeation is diffusion through the packaging material. Fick’s law of diffusion assumes the package material is not altered by the adsorption, diffusion, and desorption of a permeant such as water vapor. RH gradient and boundary conditions are established to define the boundaries at which this assumption is true or the Flory-Huggins equation is applied. For example, many packaging materials, such as ethylene vinyl alcohol, are hygroscopic, so their WVP values are reported as a function of different boundary conditions or RH gradients. This is also useful in predicting the shelf life of products with a known initial water activity and water activity at the end of product shelf life.
Decreasing Adsorption of Water Vapor
Inhibiting the adsorption of water vapor onto a package surface is more efficacious than later addressing the reduction of subsequent diffusion through the packaging. And focusing on lowering the solubility of water vapor in the package can allow for the use of packaging materials that are not commonly thought of as moisture barriers, such as paperboard. Packaging that has direct food contact, along with the outside surfaces of a package, can require resistance to water vapor. Packaged foods distributed and stored in a high moisture climate or that are in contact with liquid on a retail shelf, for example, need moisture resistance on the package exterior to ensure the structural integrity of the package. In addition, inner layers of packaging need to be highly moisture resistant when they are in direct contact with high water activity food.
Lowering the solubility of water vapor and raising the water contact angle can improve the moisture resistance of internal and external packaging layers. The water contact angle, used as a guide to measuring hydrophobicity, ranges from 72 to 102 for polyethylene, polypropylene, and polyethylene terephthalate. Materials with a water contact angle exceeding 150 are considered superhydrophobic and include waxes.
To determine the amount of water adsorbed into the surface, the Cobb test involves placing water in direct contact with the package and measuring adsorption. The basic principles of the Cobb test are often adjusted, and actual adsorption of water from food is measured for the actual shelf-life time period and temperature of contact. Correlations for accelerated conditions allow for more rapid results.
Because water adsorption aids in surface printing and adhesion, however, reverse printing on the underside of the package layer will facilitate high external moisture resistance and provide a surface suitable for printing and sealing.
Techniques for altering the package material surface to decrease water vapor adsorption include grafting, etching, deposition, and macrocoatings. Water vapor permeability of paper-based packaging, for example, is improved with chemical grafting of compounds and oxygen plasma etching to enable deposition of thin layers.
While chemical vapor deposition allows for thin coatings, more advanced methods have been adapted for packaging applications. These methods focus on enabling controlled areas of low adsorption capability on the package surface, and this allows for superior water vapor barriers, printing, and sealing. For instance, nanosurface and macrosurface roughness and low surface energy are created by a silica coating followed by chemical modification via 1H, 1H, 2H, 2H-perfluorooctyl-triethoxysilane and the deposition of polyhydroxybutyrate followed by argon plasma modification. This allows for defined and more selective water contact angles on the package surface. On a macroscale, the addition of macrosized hydrophobic curcumin to biopolyethylene and cellulose stearoyl esters on paperboard increases the water contact angle and improves barrier properties twofold.
Lipids—including wax, fatty acids, and glycerides—are insoluble in water, have extremely low solubility, and are used as package coatings, although high concentrations can present challenges with paperboard recycling processes. Fillers such as starch are added to lipid coatings to enable more consistent coatings and reduce cracking.
The most common commercial lipid coatings are triglycerides. They are used in frozen applications to reduce freezer burn and coat the inside of ice cream cones to prevent moisture gain by cones when they are filled with ice cream.
Impairing Diffusion of Water Vapor
If moisture is adsorbed on the surface of packaging, it then can diffuse through the package structure. Water vapor solubility and diffusion through metal- and glass-based food packaging is effectively zero. Technology to reduce water vapor diffusion through paperboard and polymers is well-researched and focuses on increasing the tortuous path for water vapor diffusion.
The efficacy of incorporating nanoparticles is based on their ability to retard diffusion through paperboard and polymers at low concentrations. The water vapor barrier properties of nanocomposites can be predicted using the Nielsen, constrained polymer, Maxwell, and other models that incorporate the ratio of the volume of nanoparticles to the primary packaging material as well as the aspect ratio, shape, and orientation of the nanoparticles. While the Nielsen and constrained polymer models assume that particles can be modeled as rectangles and are dispersed perpendicular to diffusion, Maxwell’s model assumes spherical nanoparticles. Although models assume no interaction between nanoparticles and the main package material, nanoparticles can alter the primary material to result in even more improved water vapor barrier properties.
Nanoparticles within packaging structures are ordered dispersion with a high surface area and large aspect ratio. Nanocomposites with aspect ratios of 100–200 improve the WVP by a factor of more than 10, while aspect ratios of 1,000–2,000 reduce WVP further.
Nanoparticles in use include carbon nanotubes, graphite, graphene, inorganic nanoparticle nanocomposites, and nanoclays. Nanoclays are commonly modified using quaternary ammonium salts to improve compatibility and interfacial interaction between the main packaging and nanoclay. Since nanoclays absorb water and swell, they are not widely used when a superior moisture barrier is needed. Hybrid polymer-clay films, such as crystalline siloxane-based lamellae, improve the water vapor barrier over 100 order of magnitude. Graphene is known to have lower permeability than clay counterparts, and 1% (by weight) of graphene can triple the WVP barrier properties of packaging materials (Xu et al. 2019).
Lipids have been used to slow the diffusion of water vapor through an edible film substrate. An abundance of research has demonstrated that the addition of lipids into packaging substrates made from edible chitosan, methyl cellulose, whey protein, pea starch, and the like can be accomplished.
However, despite the advancement of research on the incorporation of lipids into edible films via electrospinning, nanotechnology, and optimized aspect ratios, most of these edible films are not effective water vapor barriers for food. This is predominantly because the film substrate itself is very hygroscopic as well as brittle, requiring plasticizers and additives that further unfavorably impact the barrier properties. Even when lipids are dispersed within the hygroscopic substrate, the resultant water vapor barrier has limited use as a moisture barrier. WVP values are often reported in narrow water activity gradient boundary conditions that deceptively suggest the films exhibit moisture barriers similar to those of more commonly used packaging.
Since the value proposition has been unfavorable, implementation is limited. Coating ingredients within heterogeneous food systems to restrict moisture migration, however, has been successfully used for many years. The result has been food innovations with different textures within a composite food, along with extended shelf life of many heterogeneous foods, such as cheese sandwich crackers.
Designing to Control Moisture
To extend shelf life, absorption of residual moisture away from fruits and vegetables commonly is accomplished by incorporating sachets that include sodium carbonate with and without calcium hydroxide and sodium carbonate or silica gel. Moisture-absorbent pads and packaging can adsorb moisture that has wept from meat and seafood, reducing nefarious odors and microbial growth. Embedding moisture absorbers into packaging materials has been an area of much research in the past 50 years, with applications including agricultural grain packaging to reduce aflatoxin in dry corn.
Package design also plays a role in controlling moisture. Antifog packaging design reduces water droplet formation by decreasing the contact angle between the droplet and the film surface. Designs that isolate water within a separated area within a package also extend product shelf life and reduce microbial growth.
Predicting the Required Barrier
The required moisture barrier can be predicted when the allowed moisture or water activity change is known for a food product. This moisture change limit can be dictated by quality, food safety, or economic parameters. For example, the amount of moisture gain or loss allowed before a ready-to-eat cereal has an increased rate of oxidation leading to rapid rancidity is the moisture change limit in the cereal. One factor that governs moisture gain in cereal is the sugar content, and a lower sugar content cereal requires less of a moisture barrier than a high sugar cereal. Cereal with both a low sugar and a low unsaturated fat content requires less of a moisture barrier than cereal with a high sugar and a high unsaturated fat content.
The barrier requirements or shelf life can be determined using well-known static predictive models when moisture sorption isotherms that describe the relationship between the rate of moisture loss and water activity, mode of deterioration, and food moisture content are known. However, using dynamic modeling aided by computational science results in more accurate predictions.
Dynamic modeling incorporates the reality of altered RH gradients and temperature changes that occur during distribution, changing the rate of moisture loss due to food surface drying, package permeability, microbial growth, and reaction rates within foods. Critically, boundary conditions to which the permeation values apply are very relevant to food packaging. For example, ready-to-eat breakfast cereal has an initial water activity of about 0.25 and can be exposed to a variable humidity and temperatures ranging from -20°F to 80°F. If water vapor permeation values are reported at 75°F and a 0%–100% RH gradient in distribution and handling, more information is needed to perform predictive modeling. Specifically, the activation energy and Arrhenius relationship across this range of temperatures needs to be known and applied to equations (Barbosa-Cánovas 2020).
This is especially relevant because polymer films and coatings undergo a glass transition temperature phase change. Food also undergoes a phase change as the temperature changes, and this alters corresponding deteriorative reaction rates. When both package and food chemical reactions within food, as well as permeation through packaging, can be altered with temperature changes, predicting the required water vapor barrier can be complex. Mathematical models need to be built to determine a range of required water vapor barriers.
Moisture Control With Resealable Packaging
Moisture control packaging is an area of extensive research and development, focusing on enabling resealable moisture-resistant packaging and the control of moisture. Peel and reseal technology, as well as various zipper and adhesive-related efforts, have extended shelf life after the package has been opened.
Further advances are needed to enable reseal of thin packaging materials with a lower tensile strength, as well as reseal features for rigid and semirigid polymer and paperboard packaging that don’t add appreciably more packaging. This is a design innovation challenge that potentially can allow for more resealable packaging to maintain food quality and safety and reduce consumer-derived food waste.
REFERENCES
Barbosa-Cánovas, G. V., A. J. Fontana Jr., S. J. Schmidt, et al. 2020. Water Activity in Foods: Fundamentals and Applications, 2nd ed. Hoboken, N.J.: Wiley-Blackwell.
Xu, L., J. Teng, L. Li, et al. 2019. “Hydrophobic Graphene Oxide as a Promising Barrier of Water Vapor for Regenerated Cellulose Nanocomposite Films.” ACS Omega. 4(1): 509–517. doi: 10.1021/acsomega.8b02866.