Microstructure and Food Product Engineering
Food engineering can make a significant contribution to the engineering sciences by introducing structure as a fundamental variable in the study of transport phenomena and physical properties of foods. Considering structure in the design of foods is the birth of food product engineering.
Foods have a microstructure imparted either by nature or through processing. The relevance of structure in engineering is that practically all relevant properties are structure sensitive. As discussed by Aguilera and Stanley (1999), the microstructural engineer understands food processing as a controlled effort to preserve, transform, destroy, or create structures (Fig. 1).
 Preserving structure is a major objective in postharvest technology of plant material as well as in postmortem handling of fleshy tissues, since alterations in their structure lead to detrimental changes in texture, flavor, nutritional properties, and wholesomeness. Similarly, the quality of structured semisolid and liquid foods, such as gels and emulsions, needs to be maintained during distribution and storage.
Preserving structure is a major objective in postharvest technology of plant material as well as in postmortem handling of fleshy tissues, since alterations in their structure lead to detrimental changes in texture, flavor, nutritional properties, and wholesomeness. Similarly, the quality of structured semisolid and liquid foods, such as gels and emulsions, needs to be maintained during distribution and storage.
Transforming structure is central to the food processing industry. Raw materials are converted into refined ingredients that in turn are transformed into food products through unit operations involving heat, mass, and momentum transfer. Controlled destruction of structure in food processing is ubiquitous whenever reducing the size of materials is required for further processing and during mastication. Creation of structure is a major task for the food industry in this millennium. Product development and product improvement are largely based on creating matrices in which nutrients and food components are conveyed in textures and forms that appeal to the consumer.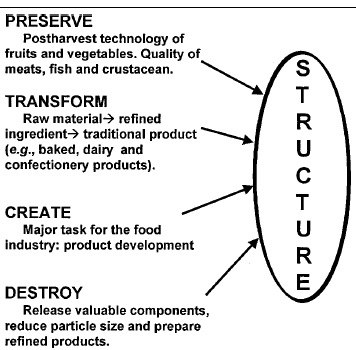
If microstructure is so important to understanding food processing, why has it been practically ignored by food engineers? There are many plausible answers, four of which follow. First, the emphasis of the food industry in the last century was on designing safe and reliable processes for large volumes of traditional products. Second, innovation and quality were not major driving forces in the food market until recently. Third, engineers knew little about the underlying sciences linking food structure to product properties. And, last but not least, techniques aiding in the study of microstructure were not developed to the point that they provided useful data (e.g., quantitative information) to engineers.
Food Product Engineering and Chemical Engineering
Major advances in food engineering during the 20th century came from transfer of knowledge from related fields such as chemical and mechanical engineering. The impact of these disciplines was largely at the macroscopic level (or macrolevel) through the adaptation of unit operations, adoption of mechanization and automation techniques, etc., which led to the transformation of an artisan production of foods into the high-volume and reliable food industry of today. This article postulates that in the coming decades materials science and biology will have a comparable impact in food product engineering and product development and that the relevant scale of analysis will shift from the macrolevel to the microlevel.
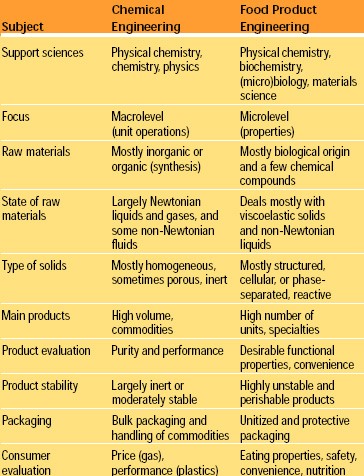
Unfortunately, the concept of structure that is fundamental in engineering sciences was largely disregarded by chemical engineers until the advent of consumer products for specialized segments (e.g., personal care products and detergents) and the emergence of bioprocessing and biomaterials. Table 1 lists some of the distinctive traits of classical chemical engineering and food product engineering from a very broad perspective. The parental influence that classical chemical engineering had on food engineering is diminishing because further advances in food fabrication will be dictated by the nature of phenomena related to materials science and biology, as will be shown below.
--- PAGE BREAK ---
The increasing role of structure in customized products from major chemical, pharmaceutical, and food industries and the lack of exposure of chemical engineering graduates to the subject was addressed in a conference with participation of representatives from industry and academia (Villadsen, 1997). It was recognized that several products from the above-mentioned industries (e.g., powders and creams) perform well in the market to the extent that they have the right structure. Participants advocated for a product engineering based on structured materials, cross-fertilization with other disciplines, application of modern techniques to study structure, and a change of emphasis in the teaching of chemical engineering.
The Microstructural Approach
Fig. 2 shows a scheme of the microstructural approach as it applies to food processing. Product engineers are aware that many properties of foods depend on their structure and understand food processing as building an “engineered” structure from available food components. This engineered structure is thermodynamically and biochemically unstable and starts to change as soon as the product leaves the production line (destructuring). However, most physical properties of these structures can be determined by standard tests (or suitable adaptations), as for any other engineered material. From the product perspective (at left in Fig. 2), each step of food processing contributes to building the desired attributes which become the quality factors in the marketplace and have to be preserved until the product is assessed by that sensitive and biased organ, the mouth.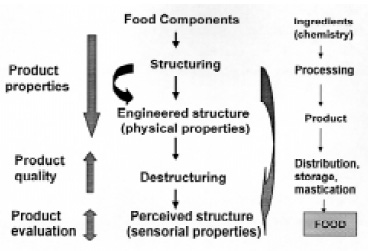
 Structure–property relationships suggest that there is a causal connection between the structure, the way a product behaves, and its engineering properties (dark arrow in Fig. 2). Product properties reflect the interactions of components and structural elements occurring at different levels in a food. The microstructural approach is fundamental to deriving and understanding structure–property relationships in food products and the basis for relating structure to perceived properties in the mouth (light arrow).
Structure–property relationships suggest that there is a causal connection between the structure, the way a product behaves, and its engineering properties (dark arrow in Fig. 2). Product properties reflect the interactions of components and structural elements occurring at different levels in a food. The microstructural approach is fundamental to deriving and understanding structure–property relationships in food products and the basis for relating structure to perceived properties in the mouth (light arrow).
Scales of Food Microstructure
Materials scientists define levels of structures to identify the scale (or scales) of critical elements and select techniques for their characterization. The relevant scale is the dimensional level at which the effects of certain phenomena are realized or explained. A first distinction is required between macrostructure (bulk or macroscopic level) and microstructure (microscopic level). As an example, Table 2 shows several decades of scales ranging from a tree to elemental components of a fruit (sugars), each one important depending on the process or engineering operation to be performed on the system. Evidently, a process may involve more than one level, and mechanisms operating at each level may be different.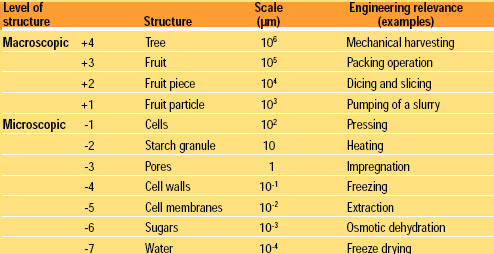
A similar analysis could be made for other food systems, but the conclusion will always be the same: the functionality of processed foods comes largely from interventions at a microstructural scale which ranges from approximately 100 μm down to 0.01 μm. This is valid also for textural properties and their sensorial perception in the mouth. In materials science, it has been known for decades that product properties are structure dependent.
--- PAGE BREAK ---
The shift of scale from process engineering to product engineering varies the approach used and the tools needed to probe materials and generate data for analysis. Chemical and mechanical engineers may feel uncomfortable working with elements critical in imparting product properties which are unseen and often unfamiliar to them: fat globules, starch granules, cell walls and membranes, air cells, crystals, gel networks, etc. Modern microscopy techniques assist in visualization of the structures and in combination with microanalytical methods and image processing may provide the needed data at the required scale (Kalab et al., 1995).
A few examples of how microstructure influences product properties and phenomena leading to their generation and transformation—desired or unwanted—as well as means of characterizing them are presented below.
Structure and Transport Phenomena
Transport phenomena and their quantitative modeling are central to food engineering. According to Bailey (1998), modeling does not make sense without defining beforehand what is intended to be resolved. Modeling should represent and interpret how things work, bring order to experience, clarify important interactions, and generate new hypotheses. How is it then possible that food engineers have devoted so little attention to the physical model in the study of transport phenomena, instead favoring the “black box” approach?
Transport phenomena in foods and biological materials depend strongly on structure. Food engineering could make a significant contribution to the engineering sciences by introducing structure as a fundamental variable in the study of transport phenomena. Structures should be visualized by the best available microscopy techniques and used to derive realistic physical models on which basic equations and computational methods are applied.
Fick’s laws for molecular diffusion are extensively used by engineers to model mass transport in foods, often disregarding some severe restrictions that their application imposes on the system under study (Gekas, 1992). In these equations, the effect of structure and structural changes in the food matrix is conveniently hidden in the so-called effective diffusion coefficient, which may vary by several orders of magnitude during processing.
There are cases, however, where mass transfer does not occur by diffusion from the interior of a matrix and where structure continues to play a key role. One such case is washing during solvent extraction. Size reduction of cellular material necessary to achieve faster extraction rates (diffusion rates vary with the square of dimension) produces broken and damaged surface cells in pieces and the instantaneous release of their content to the solvent medium. If the solvent is particularly selective for the extract, then structure can be obliterated to achieve faster and more complete extraction without impairing the downstream purification steps (e.g., oil extraction with hexane). However, when this is not the case (i.e., in most aqueous extractions of biological materials), structure needs to be damaged only to the extent that facilitates the release of the desired product (e.g., sucrose extraction from sugar cane).
A unique case of mass transfer where Fickian diffusion is secondary is moisture release and oil uptake in potato strips during deep-fat frying. Frying involves loss of water from the piece and impregnation of oil into a thin outer layer or crust. Water leaves the outer layers of cells as steam bubbles (a form of bulk transport). After removal from the fryer, most of the oil in the fried product wets the surface of the piece, while some of it has penetrated into crevices and pores in the outer cell layers. Upon cooling, wetting oil is suctioned into intercellular spaces by a negative pressure due to condensation of steam in the interior of the crust. Confocal laser scanning microscopy (CLSM) has been instrumental in revealing with minimal intrusion and large 3-D resolution that cells in the crust of a fried potato strip remain largely intact and oil-free, while oil impregnating the crust takes an egg-box arrangement surrounding these cells (Pedreschi et al., 1999). This intercellular oil is easily exchanged if the fried product is fried again in another oil (Fig. 3).
--- PAGE BREAK ---
Mechanical Properties
Nowhere is the role of structure more germane to engineers than in its effects on mechanical properties and, by extension to foods, on texture. Foods having similar chemical composition may exhibit totally different mechanical behavior depending on the structure that has been imparted through processing (Aguilera and Stanley, 1999). Although mechanical models are available for composites and cellular materials, their application to foods requires that a structure and phase fractions be determined beforehand, and here is where microscopy plays a key role.
Air and water are two ingredients that need to be “structured.” Aerated foods are thermodynamically unstable, and air bubbles need to be encased in a viscous liquid phase or a viscoelastic matrix (Campbell and Mougeot, 1999). Gels are a preferred way used by food technologists to “solidify” large amounts of water at room temperature. Since nonlinearity in structure–property relationships of composite or cellular gels arises largely from the architecture of the microstructure, its effect on mechanical properties of composite gels will be used as an example.
Starch suspensions and some protein solutions may form gels after heating, protein gels being much stronger at equal concentration. In particular, tuber starches and whey proteins undergo significant order–disorder transitions when heated in the presence of water at temperatures above 60 and 75°C, respectively. Upon cooling, an ordered structure is achieved in the form of macromolecular networks called gels. Starch-filled protein gels are composites which exhibit a compressive strength that depends on the ratio of the starch and protein phases but more importantly on the kinetics of gelation (Aguilera and Baffico, 1997). As the mixture of starch and whey proteins is heated, starch granules become hydrated, swell, and “gelatinize” at 65–70°C, removing water from the system and separating out as a distinct phase. When the gelation temperature for whey proteins is reached (ca. 75°C), they denature and form a network of aggregates from a more concentrated solution. Depending on the relative proportions of each phase, the resulting composite gels may have a compressive strength higher or lower than that of the pure protein gel (at equal total solids content). These filled gels show enhanced mechanical and rheological properties at low volume fractions of starch, although the tarch gel itself has extremely poor mechanical properties. If the volume fraction of starch is low enough so that a strong continuous network of the gelling protein is formed around hydrated starch granules, the compressive strength of the filled gel will be higher than that of the pure protein (Fig. 4). However, when the starch fraction increases, phase inversion occurs and starch becomes the continuous phase of the mixed gel. As a consequence, the mechanical properties of the gel decrease. Video microscopy of the process provides instant images of structure formation as well as the final form of the composite gel, from which volume fractions may be estimated and standard equations for mechanical properties of composites applied.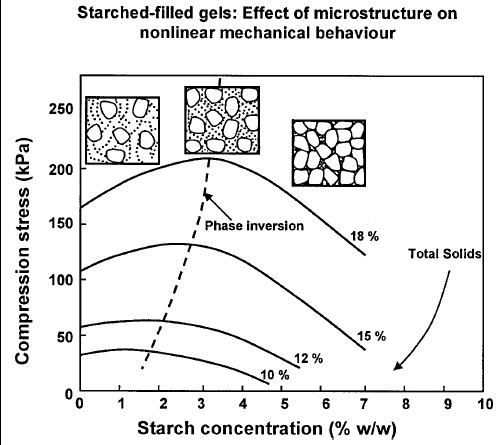
This example (and others) suggests a trend that studies on structuring of food products may follow in the future. It starts with a comprehension of the main mechanisms leading to structure formation studied by the most appropriate microscopy techniques and supported by other experimental data (e.g., rheology or differential scanning calorimetry, DSC). Then, mathematical models should be derived based on available structural data and previous findings that allow prediction of a property for any change in structure (e.g., those induced by a change in formulation).
--- PAGE BREAK ---
Product Rheology
Turning now to foods that flow, it may be hard to think that there is a “structure” in flowing systems, but deviations from ideal Newtonian behavior can always be traced back to structural aspects of the dispersed elements (i.e., macromolecules, liquid or solid particles) in the solvent, and to their interactions. Rheology is extremely important in the design of food engineering operations, and in practice the engineer is confronted with multiple equations and dimensionless numbers that depend on the viscosity.
Most complex liquid foods are non-Newtonian fluids, such as two-phase liquid systems (emulsions), biopolymer solutions, particle suspensions, etc. Non-Newtonian behavior is characterized through a single parameter called the apparent shear viscosity that takes different values depending on the local shear rate. In other words, in these fluids the instantaneous and local “structure” of the fluid determines its viscosity or resistance to flow, not to mention some time effects. Since interaction forces are potential forces, which are therefore elastic in nature, viscoelastic responses are also observed. Discussion of structural effects in suspensions is found in Macosko (1994), and the general topic of rheology of foods was recently addressed by Rao (1999).
All effects leading to nonlinear rheological behavior in nondilute systems (and sometimes in dilute systems as well) have a structural component. These can be hydrodynamic effects due to the modification of the velocity distribution field, Brownian motion, change in shape or even breakup of droplets, particle shape and orientation, interparticle forces, and entanglements or segment-to-segment contacts in the case of semi-concentrated and concentrated polymeric solutions.
In summary, products that behave as complex fluids possess a structure that varies with flow conditions and time and determines their rheological properties. Structural models should become increasingly used in rheology, the idea being that rheological behavior is the manifestation of the contribution of each element to flow and of their interactions. Hence, observation of structures at the molecular and supramolecular level during flow should be correlated with rheological data to derive functional models (Windhab, 1995). Such an approach may have a large impact in product development and product improvement, as well as in formulation and ingredient substitution.
Microstructure and Phase Stability
At the microstructural level, most solid foods are multidomain systems containing several phases. These phases are not in thermodynamic equilibrium, and migration of components (most notably moisture) may occur among phases and between a phase and the environment. Data used to predict stability of foods (i.e., water activity or glass transition temperature, Tg) are average or overall values determined from bulk samples by standard analytical procedures.
Thanks to advanced microprobe techniques that combine microscopy and spectroscopy, it is now possible to localize and map the composition and state of individual phases in a particular area in the field of view. Raman microprobes can detect with high spatial resolution (1 μm) proteins, lipids, and carbohydrates in the presence of water as well as distinctive phases of the same component (i.e., polymorphs, amorphous and crystalline states, etc.). Infrared microprobes have been used to study plant cell walls and crystallization of amylopectin (Wilson, 1995).
Many foods are stabilized at low moisture contents below their Tg as metastable, non-equilibrium amorphous or glassy solids. Chemical inertness and structural stability are based on the extremely low mobility of the glassy state (viscosity ca. 1,013 Pa.sec). Departure from the glassy state is usually detrimental to product quality. Typical examples of materials stabilized in the amorphous state are dried and frozen foods, baked products, freeze-dried bioproducts, and encapsulated ingredients.
--- PAGE BREAK ---
Sugars in confectionery, baked, or dried food products may exist in a rubbery state that tends to the thermodynamically stable crystalline form at rates that depend on temperature, moisture content, and time (Roos, 1994). Often, the presence of crystallinity is detrimental to the acceptance of the final product, as is the case of lactose in some dairy products and sucrose in boiled sweets. The rubber–crystalline transition produces an exotherm in DSC, while in polymer science the growth of crystals is directly observed and measured by polarized microscopy. DSC is prone to artifacts (e.g., nucleation on the walls of the sample pan), it is invasive,and the transition cannot be unambiguously ascribed to one component.
Miniaturization of processes under the microscope tends to reproduce moisture and temperature conditions in a microenvironment surrounding a sample, while the evolution of structural changes over time is monitored with a videocamera. The use of a “hot-stage” attached to a microscope where heating/cooling cycles can be programmed and temperature/time recorded continuously is common in biology to study freezing/thawing of cells. Fig. 5 shows a gallery of images obtained using polarized light microscopy of the kinetics of growth of individual sucrose crystals under constant moisture content and temperature. The rate of crystallization can be quantified using image analysis by changes in area and/or dimension of the crystals with time and modeled by the Avrami equation. The added value of microscopy is that the shape and pattern of crystal growth can be observed and quantified as well.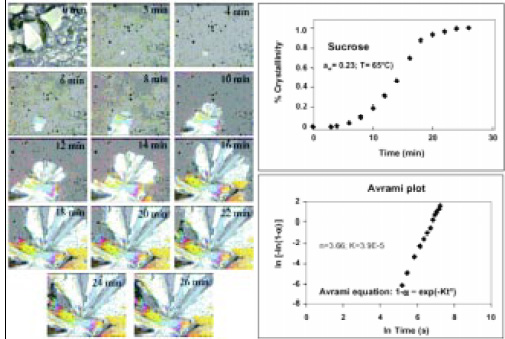
Structure of Surfaces
Surface topography or surface morphology is an important physical property of solid products, influencing not only their visual and sensory aspects but also their behavior during processing, storage, and use. Surface topography is related to the specific surface area on which chemical and physical interactions may occur. Moreover, from a marketing viewpoint, the first impression a consumer gets from a product is that of its surface. Important as it may seem, surface characterization has not been addressed to a major extent by food engineers.
A surface that looks smooth to the naked eye may be quite rough when observed under a simple microscope, and this roughness may have technological implications. For instance, the brain may interpret as “sandy” surfaces that expose to the tongue particles on the order of 30–50 μm, and the eye may perceive as dull the surface of chocolate having minute fat crystals. Consequently, there is interest in determining at which scale the smooth-to-rough transition occurs and, below this point, how rough the surface is.
Material scientists have successfully characterized surfaces in an ample range of dimensions to understand topographically related phenomena in engineering materials. Recent advances in techniques for acquisition of surface data—e.g. scanning laser profilometry system (SLPS), interferometry, atomic probe, and confocal scanning microscopy, among others—generate heights (z) as a function of position (x, y) at high levels of resolution (e.g., 1 μm or less). The process is completed by performing area-scale analysis using the patchwork method to determine apparent areas of surfaces over a range of scales by repeated virtual tiling exercises (Brown et al., 1993). After a sufficient number of iterations of the tiling procedure, the result is expressed as a log-log plot of relative area (apparent area divided by the projected area) vs the area (scale) of the triangular patch.
This scale-sensitive area analysis of surfaces allows the calculation of two characterization parameters. One parameter is the smooth–rough crossover scale (SRC), which is the scale above which the surface appears smooth; it is easily characterized by Euclidean geometry. Below the SRC, the surface appears rough and can be characterized by fractal geometry. Fig. 6 shows that the relative areas are close to 1 at scales above the SRC, and markedly greater than 1 below the SRC. The SRC has a clear physical interpretation in that interactions at scales above the SRC see the surface as smooth, while interactions at scales below the SRC see it as rough.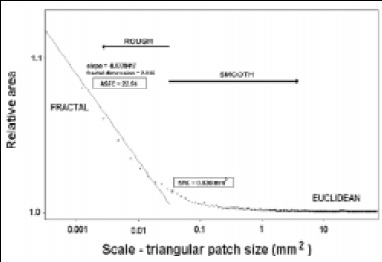
--- PAGE BREAK ---
The other parameter is complexity, which for area-scale analysis is the area-scale fractal complexity (ASFC). The complexity parameter is equal to –1,000 times the slope of the respective log-log area plots. It is related to the fractal dimension, which is 2 minus the slope of the area-scale plot. A large ASFC is an indication of higher complexity, intricacy, or roughness of the surface at scales below the SRC. If the data exhibit multifractal behavior, there may be different slopes that describe the plot over different scale ranges.
What Needs to Be Done
In the past, the application of physics and classical principles of chemical engineering, combined with a certain dose of empiricism, were sufficient to explain phenomena in food processing at the macrolevel. However, this approach has led to a limited contribution to the fundamental principles of food product engineering, sometimes justified by the “complexity” of food materials, the reduced scale of analysis, and the difficulty of generating quantitative data at the microlevel.
Structure has been a most important variable disregarded by food engineers. For further advances in the understanding of properties of food products as well as advances in processing and fabrication, we need to reduce the scale of analysis to that of basic building elements and the cell. The myriad of microscopy, visualization, and image processing/analysis techniques available, including noninvasive and real-time methods, will greatly assist in this effort. Food products must also be understood in the way they are formed, transformed, and broken down in the mouth.
This trend will be greatly assisted by concepts, techniques, and tools borrowed from biology and materials sciences. Food engineers should be trained at universities and industries along these lines that tacitly imply multidisciplinary and team work. Product fabrication or creating structures de novo may also be a way of conveying nutrients and improving the safety of foods.
Research at the Biomaterials Laboratory of the Pontificia Universidad Católica de Chile was supported by grants from Fondecyt, the Nestlé Research Center, Vers-chezles-Blancs, Switzerland; the J.S. Guggenheim Foundation, New York; and the A. von Humboldt Foundation, Germany. Nestlé-Chile provided financial support for the graduate studies of F. Pedreschi.
by JOSÉ MIGUEL AGUILERA
The author is Professor of Chemical and Food Engineering, Dept. of Chemical and Bioprocessing Engineering, and Associate Dean, College of Engineering, Pontificia Universidad Católica de Chile, P.O. Box 306, Santiago, Chile.
Edited by Neil H. Mermelstein, Senior Editor
References
Aguilera, J.M. and Stanley, D.W. 1999. “Microstructural Principles of Food Processing and Engineering,” 2nd ed. Aspen Publishing Co., Gaithersburg, Md.
Aguilera, J.M. and Baffico, P. 1997. Structure-mechanical properties of heat-induced whey protein/starch gels, J. Food Sci. 62: 1048-1053, 1066.
Bailey, J.E. 1998. Mathematical modeling and analysis in biochemical engineering: Past accomplishments and future opportunities, Biotechnol. Progr. 14: 8-20.
Brown, C.A., Charles, P.D. Johnsen, W.A., and Chesters, S. 1993. Fractal analysis of topographic data by the patchwork method, Wear 161: 61-61.
Campbell, G.M., and Mougeot, E. 1999. Creation and characterization of aerated food products. Trends Food Sci. Technol. 10: 283-296.
Gekas, V. 1992. “Transport Phenomena in Foods and Biological Materials.” CRC Press, Boca Raton, Fla.
Kalab, M., Allan-Wojtas, P., and Shea Miller, S. 1995. Microscopy and other imaging techniques in food structure analysis. Trends Food Sci. Technol. 6: 177-186.
Macosko, C.W. 1994. “Rheology: Principles, Measurements and Applications.” VCH Publishers, New York.
Pedreschi, F., Aguilera, J.M., and Brown, C.A. 2000. Characterization of food surfaces using scale-sensitive fractal analysis, J. Food Proc. Eng. 23: 127-143.
Pedreschi, F., Aguilera, J.M., and Arbildua, J.J. 1999. CLSM study of oil location in fried potato slices. Microscopy Analysis 37: 21-22.
Rao, M.A. 1999. “Rheology of Fluid and Semisolid Foods.” Aspen Publishing Co., Gaithersburg, Md.
Roos, Y. 1994. “Phase Transitions in Foods.” Academic Press, New York.
Villadsen, J. 1997. Putting structure into chemical engineering. Chem. Eng. Sci. 52: 2857-2864.
Wilson, R.H. 1995. Recent developments in IR spectroscopy and microscopy. In “Physicochemical Aspects of Food Processing,” ed. S.T. Beckett, pp. 177-195. Aspen Publishing Co., Gaithersburg, Md.
Windhab, E. J. 1995. Rheology in food processing. In “Physicochemical Aspects of Food Processing,” ed. S.T. Beckett, pp. 80-116. Aspen Publishing Co., Gaithersburg, Md.
