Reflections on Salmonella and Other “Wee Beasties” in Foods
This year’s IFT Food Microbiology Division Lecturer discusses the role that microbial biofilms may have in foodborne disease outbreaks and the importance of cleaning and sanitation in their control.
When I took my first course in food microbiology more than 45 years ago, the lectures related to foodborne disease where only a minor part of the course. Outbreaks of foodborne disease were considered to be a problem related to the final preparation of the food in the home, restaurant, or institution. Those outbreaks that did occur were small in comparison to what we see today.
Foodborne Outbreaks through the Decades
Here’s what happened in the years since then:
• The ‘60s—Decade of Awakening. It was in the 1960s that we began to see major outbreaks of foodborne disease associated with processed foods. Regulatory agencies were becoming increasingly aware of the dissemination of pathogenic bacteria with processed foods. One of the first out-breaks involved Salmonella derby and poultry products and encompassed most of the East Coast of the United States (Sanders et al., 1965). 1964?
While this outbreak was being discussed, dissected, and conferenced, another unusual serotype of Salmonella, Salmonella new-brunswick, showed up as the causative organism in a number of cases of salmonellosis (Collins et al., 1966). The number of cases involved was small (26), but they involved infants and an unusual serotype, so further investigation was done. The results identified nonfat dry milk (NFDM) in an infant formula as the contaminated food.
Noteworthy things about this outbreak were that a highly processed food that did not contain eggs was the culprit. This in turn triggered a frenzy of testing of NFDM for the presence of Salmonella. It was quickly discovered that a significant amount of NFDM in the marketplace at the time was contaminated with many different Salmonella serotypes. In addition, the environments of a large number of processing plants producing this product were contaminated with Salmonella. The problems were so great that several manufacturers closed their plants rather than try to resolve the problem.
The activities of the regulatory agencies involved with this problem awakened the food industry to the probability that they had better be prepared to deal with Salmonella and the potential for contamination of their products with this and other “wee beasties” (as Antony van Leeuwenhoek called the organisms that he first saw in his new microscope).
The National Academy of Sciences in 1969 (NAS, 1969) published an evaluation of the Salmonella problem, which outlined the concepts that eventually resulted in the development of the Hazard Analysis Critical Control Point (HACCP) system. As a result of all of this, control procedures that are still in effect today were established. In most instances, they controlled the problem, but in some others they did not.
• The ‘70s—Decade of Discovery and Change. In the ‘70s, rapid methods for determining the presence of Salmonella in foods were developed. The Pillsbury Co. initiated the concept of HACCP, which was put into practice for the manufacture of foods for the space program (Pillsbury, 1973). Two outbreaks of botulism in the early ’70s resulted in two deaths from two different commercially canned foods. The canned foods had not been properly heat processed (Zottola, 1976). To resolve this problem, the Food and Drug Administration instituted strict Good Manufacturing Practices (GMPs) regulations (21 CFR, Part 110) with stiff penalties for the canning industry to resolve this problem.
--- PAGE BREAK ---
• The ‘80s—Decade of Listeria Hysteria. The ‘80s introduced us to a new foodborne pathogen, Listeria monocytogenes. The food involved was a soft white cheese favored in Southern California. The arrival of this new problem organism was a total surprise to the food processing industry and to most food microbiologists. Investigation of other prepared foods indicated widespread contamination in ice cream, processed meats, prepared sandwiches, and other refrigerated foods. L. monocytogenes is a unique organism in that it is able to survive and grow at temperatures lower than most other pathogenic organisms (Lovett, 1989). The industry and regulatory agencies once again attempted to develop programs to prevent contamination of food with this insidious organism. Sometimes they worked, other times they didn’t.
Salmonella reappeared as a major problem in 1985. A massive outbreak in Illinois was caused by pasteurized milk (Ryan et al., 1987) contaminated by a unique strain of Salmonella typhimurium. The strain was unique because it was resistant to 14 different antibiotics (Schuman et al., 1989). The source of this strain was thought to be raw milk that contaminated the pasteurized milk. There were more than 200,000 cases associated with this outbreak.
One other outbreak that occurred in the ‘80s that would eventually have a major effect on the food industry was caused by Escherichia coli O157:H7 in undercooked hamburgers served in two fast food restaurants (Riley et al., 1983, CDC, 1982).
• The ‘90s—Decade of E. coli O157:H7. The decade of the ‘90s involved the emergence of E. coli O157:H7 as a cause of foodborne illness. It quickly became the dominant concern in food safety and food microbiology. Undercooked hamburgers put out by a fastfood chain in the Pacific Northwest caused the outbreak that brought this organism to the top of the research tower. More than 500 cases and several deaths were associated with this outbreak (CDC, 1993). There were a number of other outbreaks in the late ’80s and the ‘90s that were caused by E. coli O157:H7, but it was the one associated with the undercooked hamburgers that resulted in increased regulatory activity. Over the past 10 years, millions of dollars worth of hamburger contaminated with E. coli O157:H7 have been recalled and destroyed.
L. monocytogenes also continued to be a major concern, with several recalls of processed ready-to-eat foods during this decade. But the bacterium that caused the greatest number of sick people was once again Salmonella! An outbreak of salmonellosis associated with home-delivered ice cream contaminated with Salmonella enteritidis caused an estimated 250,000 cases (Hennessey et al., 1996). A second outbreak of salmonellosis was caused by Salmonella agona- contamination of dry breakfast cereal, another ready-to-eat food considered by many to be inert to microbial contamination (CDC, 1998).
No matter how hard we try, Salmonella and other pathogens keep causing major outbreaks of foodborne illness. We give them opportunities to come back! A good example of how these opportunities occur is a recent outbreak of salmonellosis that occurred in May 2001 in Minneapolis. Raw eggs used in preparing the Hollandaise sauce for Eggs Benedict served at buffet at a major hotel was the culprit. The newspaper account of the outbreak indicated that 46 of those attending the buffet became ill. In addition, a chef, eight hotel engineers, and at least two waiters became ill.
Role of Microbial Biofilms
In the remainder of this article, I will focus on the role that microbial attachment and the development of biofilms in or on food processing equipment may have had on the contamination of the foods involved in these outbreaks of foodborne illness.
What is a microbial biofilm? Many definitions have been put forth, but the one that will be used here is that suggested by Marshall (1992). Microbial biofilms consist of microorganisms immobilized at a substratum surface, usually embedded in an organic polymer matrix of bacterial origin. Such biofilms are ubiquitous in flowing environments, are not necessarily uniform in time and space, and may trap inorganic substances within the polymer matrix. Mature biofilms such as described in this definition require 2–4 weeks to develop (Marshall, 1992). This last statement is critical to understanding biofilm formation in the food processing industry.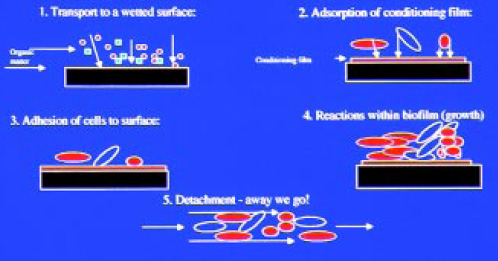
--- PAGE BREAK ---
How do biofilms form? The five-step theory of biofilm formation as proposed by Characklis and Cooksey (1983) fits both the definition given above and the two-step theory of initial reversible adherence and irreversible adherence as the biofilm develops (Marshall et al., 1971). This definition takes into account physical, chemical, and biological phenomena. The five stages suggested (Fig. 1) are:
1. Transport of nutrients, inorganic matter, and organic matter to the solid surface.
2. Adsorption of a conditioning film containing inorganic or organic nutrients.
3. Attachment of microbial cells to the conditioned surface, and initiation of growth.
4. Bacterial metabolism resulting in growth within the biofilm.
5. Cell disruption and detachment from the biofilm.
A major problem in the study of biofilms is the inability to study them as they develop in a natural setting. Election microscopy techniques have been used in the past, but these have severe limitations. Recent advances in electron microscopy equipment and methods have removed some of the frustration associated with earlier instruments, procedures, and interpretation of results. Confocal scanning laser microscopy (CSLM) is one of the new instruments, and the development of procedures to study biofilms with this instrument has given new information on the structure of microbial biofilms (Zottola, 1997).
The Center for Biofilm Engineering at Montana State University developed a conceptual model of a biofilm based on CLSM. They were able to observe living, fully hydrated microbial biofilms free of artifacts commonly seen in other forms of electron microscopy. A representation of this model is shown in Fig. 2. Their studies showed that bacterial cells were located in discrete microcoloniesembedded in a matrix permeated by well-defined channels. Analysis of the structure has indicated bacterial colonies 20–50 microns within the biofilm; thus, the colonies are able to maintain contact with the surface and moisture passing through the channels to maintain biological activity (Anonymous, 1994).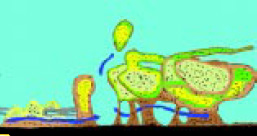
Several investigators have established that microbes usually associated with foods are able to adhere to food contact and non-food contact surfaces. Work done in our laboratory t the University of Minnesota has shown that L. monocytogenes, Salmonella montevideo, E. coli O157:H7, Yersinia enterocolitica, and Pseudomonas fragi readily adhere to these surfaces (Schwach and Zottola, 1984; Herald and Zottola, 1988; Sasahara and Zottola, 1993). It has also been observed that P. fragi grown with L. monocytogenes will develop a more complex biofilm than when grown singly (Fig. 3). Using transmission electron microscopy and heavy metal staining, Sasahara and Zottola (1993) showed that P. fragi produced an exopolysaccharide material that is involved in adherence by this organism to stainless steel. This exopolysaccharide was not found when the same procedures were used with L. monocytogenes. This suggests that the adherence of L. monocytogenes is reversible, while adherence of P. fragi is not. When the two are grown together, they coexist or grow symbiotically, forming a complex biofilm, as suggested in Fig. 2 and shown in Fig. 3, which is not reversible. 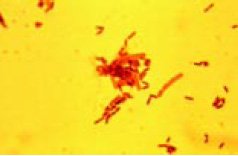
--- PAGE BREAK ---
Do such complex microbial biofilms exist in food processing systems? I think that they do, as all the conditions exist for the development. To grow, microorganisms need moisture, nutrients, and favorable temperature, atmosphere, and pH. As is well known, microbes are very small and take up very little space; thus, the “growth niche” needed for the formation of a biofilm is also a very small space. Growth niche is defined as an area that contains all the needed requirements for microbial growth that allows unlimited growth. In food processing systems, growth niches are almost everywhere. Consequently, the opportunity for the development of complex biofilms does exist!
As we learn more about microbial biofilms and the potential for these biofilms to be associated with food processing, it might be necessary for us to change our thoughts about how bacteria act or react in a food processing environment. They don’t do what we think they should do! I frequently tell those concerned about microbial contamination that they have to “think like a bacterium” to better understand them, why they do what they do, and what they are doing.
It is also important to remember that these “wee beasties” that make us sick, that cause spoilage problems, carry out fermentations, and so on were here long before we were. They will be here long after we are gone. They can be heated, frozen, dried, squeezed, acidified, sanitized, and reprogrammed, yet they still come back for more. They are indeed survivors.
Where might biofilms be in a food processing system? How about: weep holes, stress cracks in jacketed vessels, walls covered with glass board, dead ends, heat exchangers, water cooling systems, glycol cooling systems, sewer traps, the underside of process equipment, conveyors and transfer chain systems, bag houses, wet collectors, cooling coils, drip pans, overhead transfer systems, etc. Anywhere there is a growth niche, biofilms will eventually form. The trick is to find and prevent the formation of growth niches.
How do microbes find these growth niches? There are many possible mechanisms: aerosols, stress cracks—caused by fluctuating pressures—in walls covered with other materials such as stainless steel or glass board, jacketed vessels, and heat exchangers, dirty equipment, wastewater, human contamination, maintenance, product waste, and so on. All most any method you can think of will result in microbial transfer to growth niches. If there is an opening, the microbes will find it.
--- PAGE BREAK ---
Are microbial biofilms involved in this transfer? You bet! If Fig.2 is a true representation of what a microbial biofilm looks like in a food processing system, then transfer of cells to another site for biofilm formation readily occurs. Note that in approximately the center of the diagram, a piece of the biofilm has broken away and is in the stream flow on its way to another site.
The term “biofilm” has been used exclusively in this article to describe adherent microbes in the food processing environment. This may be confusing, as there have not been any published reports that have positively identified mature biofilms in this environment. Several papers have been published that indicate that microbes associated with foods are able to adhere to food-contact and non–food-contact surfaces. Food processors have to be concerned because there is a zero tolerance for L. monocytogenes, Salmonella species, and E. coli O157:H7 in foods. Thus, a single adherent bacterium of these three is cause for concern.
Biotransfer Potential
I stated above that it takes 2–4 weeks for a classical biofilm, as shown in Fig. 2, to form. Classical biofilms would only form in food processing systems where cleaning and sanitation practices are deficient, thus allowing formation. It would be the exception rather than an actual occurrence that classical biofilms would form in food processing environments where proper cleaning and sanitizing practices are followed. But initial adherence would occur, and initiation of growth would begin. Thus, our concerns may not be related to controlling biofilms but to controlling adherence.
To address this concern, Hood and Zottola (1995) developed the concept of “biotransfer potential.” This concept may be more appropriate when discussing this problem in the food processing industry. Biotransfer potential describes any adherent microorganisms in food processing associated with a surface that could eventually lead to contamination of the food in contact with the surface. In essence, what this implies is that the greater the number of microbes adhering to the surface, the greater the potential for product contamination.
Role of Biofilms in Food Contamination
Were microbial biofilms involved in the outbreaks of salmonellosis described earlier? Let’s pretend we know what happened. If biofilms are involved, then contamination would be sporadic, not continuous, as pieces of the biofilm break off periodically. In each of the outbreaks discussed above, only portions of the products were contaminated, not all of them. If the source of the contaminant was a liquid system, then all of the product would contain the pathogenic organism.
In the 1965 outbreak of salmonellosis associated with NFDM, the actual source of the Salmonella was never identified. But the processing system included an instantizer which was suspect. The instantizer was infrequently wet-cleaned, providing a growth niche somewhere in the equipment. Thus, the potential for biofilm existed, and contamination resulted. In the pasteurized milk outbreak, a crossover pipe containing raw milk was implicated as the source of the Salmonella, but no salmonellae were isolated from the pipe or anywhere else in the plant. Since contamination was random and low-level, biofilms could have been involved. Mix used in the outbreak associated with ice cream was transported in a tanker that had been used to haul pasteurized eggs and was thought to be the source of the contamination. But again, product contamination was random, not suggesting that biofilms were involved. It’s too late to confirm these possibilities, but it should give us something to think about.
--- PAGE BREAK ---
If one were to look at the many outbreaks and recalls associated with Listeria, I feel that similar conclusions could be made. For example, in the original outbreak of listeriosis caused by white cheese, a source of the Listeria was never found. Raw milk contamination of the pasteurized milk was proposed, but it could have been the cooling system used with the high-temperature, short-time equipment. Research done in our laboratory (Petran and Zottola, 1988) demonstrated that Listeria survive in cooling-water systems and in up to 30% glycol systems. If the pressures in the cooling section of the HTST were not properly maintained, leakage from the cooling water into the pasteurized milk could occur. Biofilms of Listeria and other microbes could have been in the cooling system.
Sources of E. coli O157:H7 in foods may be related to biofilms of a different nature. Microbes attach to and get trapped in meat fibers. Fig. 4 is a scanning electron micrograph of the surface of a piece of beef with organisms attached or adhering to the meat fibers. Note that it is difficult to tell if the meat fibers are holding the cells or if the cells are sticking to the meat fibers (Schwach and Zottola, 1982). When the meat is ground, the cells get well distributed in the ground meat. This provides even more sites for biofilm development and increases the potential for more E. coli O157:H7 to be available for consumption. Additional research should be done on the attachment and detachment of this insidious organism in meat and ground meat. A better understanding of how this organism resides in the meat complex would assist in developing better control procedures. 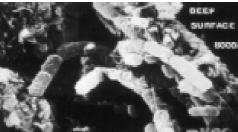
Preventing Biofilms
What’s involved in preventing microbial biofilms in food processing systems? It’s simple. Proper cleaning and sanitizing programs for equipment and non-food-contact surfaces. I have been preaching this for years, and a few have been converted. Those plants that have given top priority to cleaning and sanitizing (which are used interchangeably here) have very few contamination problems. There is no easy solution.
The chemistry and microbiology associated with cleaning and sanitizing is not a very glamorous topic on which to do research. It is way down on the priority list of current topics for research in most university settings. Yet, if one thinks about it, proper cleaning and sanitizing is the backbone of food processing. Without proper cleaning and sanitizing, there will be major spoilage problems and outbreaks of foodborne disease. As a result, there will be no jobs for marketing geniuses, chief executive officers, plant managers, quality assurance directors, and so on, because the company will be out of business.
The control of microbial biofilms in food processing is the development of good sound cleaning and sanitation programs. This would include the development of training programs, sufficient time to clean the equipment and the plant, higher priority in upper management, and required higher educational level for cleaning crews. Equipment is becoming more complicated and difficult to clean. Management is directed at long runs to get more products through the system in the shortest possible time. Systems are frequently operated for very long periods between cleanings, in excess of 18–24 hours, sometimes days or even weeks! Production lines are bigger, run at higher speeds, and have more maintenance requirements. Downtime for cleaning is usually late night or early morning, when upper management and sometimes no management is present. Food processing plants are getting bigger, and distribution systems now cover the entire United States and other parts of the world. Food companies are huge conglomerates that produce many different products. As a result, outbreaks of foodborne disease are bigger (providing job security for food microbiologists): whereas the 1965 outbreak associated with nonfat dry milk contaminated with S. new-brunswick had 26 cases, the 1994 outbreak caused by ice cream contaminated with S. enteritidis involved more than 250,000 cases—a considerable difference!
--- PAGE BREAK ---
Proper cleaning and sanitizing will assist in controlling microbial biofilms, but other things also need to be done. Engineers involved in the design of food processing equipment, plants, and product flow in these plants have to develop an understanding of microbiology. With this knowledge, they then should be able to design equipment, processing plants, and the plant environment to eliminate microbial growth niches. Is it possible to develop foodcontact surfaces so that the initial adherence or attachment is prevented? Would this assist in preventing biofilm development?
There is a need for better training of sanitation personnel so that they understand what they are doing and why they are doing it. Proper cleaning procedures, with sufficient time and frequency of cleaning, will control microbial biofilms. To effectively control biofilms in any food processing environment, be it wet or dry, requires a much higher priority than it now has.
Additional research has to be directed at developing a better understanding of microbial biofilms formation and disruption in food processing systems and environments. There are still too many unknowns about microbial biofilms and their role in the contamination of food by spoilage and pathogenic microbes. A better understanding will be attained when we are able to answer such questions as: Do classical microbial biofilms form in food processing systems? How long does it take for this to occur? What effect does biofilm formation have on food-contact surfaces? Will it change the surface in any way? What are the critical growth conditions needed for microbial growth? Can these be used to control or prevent biofilm formation? Are there better methods than those now available for cleaning and sanitation to control biofilm formation? These are but a few of the questions that need to be addressed to help us better understand the formation and control of biofilms. The Center for Biofilm Engineering at Montana State University concentrates on the study of microbial biofilms in other industries, such as oil, medicine, water systems, medical devices, hazardous waste, and so on, but little attention, if any, is paid to the food processing industry. Would a “Center for the Study of Microbial Biofilms in Food” be of use to us?
Somethings to Think About
I would like to conclude with a dozen “ions” to think about and use in the control of biotransfer potential:
Communication—Talk about your problems with your employees.
Cooperation—Work together to resolve your problems.
Sanitation—Practice it in all facets of your life.
Education—Teach/train your employees.
Elimination—Keep your system clean and sanitary to eliminate microbial biofilms.
Concentration—Concentrate your efforts on controlling microrganisms and biofilm development.
Verification—Make sure every person is doing his or her job correctly.
Certification—Develop a program to recognize those who do their jobs well, and certify that they have done it well.
Justification—Tell your employees why they have to do something, not simply that they have to do it!
Pasteurization—Use it to control non-sporeforming problem organisms.
Sterilization—Use it to control spore-forming problem organisms.
Radiation—When all else fails, irradiate!
In closing, I would like to share with you some thoughts about these problems with foodborne disease and what may lie ahead. If we look at the occurrence of outbreaks of salmonellosis as we do the growth curve for bacteria, then we could put the initial 1965 outbreak involving 26 cases as the lag phase, and the 1985 outbreak involving 200,000 cases and the 1994 outbreak with 250,000 cases could be the beginning of log growth. Will the log phase continue to accelerate until we experience one outbreak with 1 million cases? If the food processing industry fails to put higher priority and greater emphasis on cleaning and sanitation, we may indeed see such an outbreak.
Based on the Food Microbiology Division Lecture at the Annual Meeting of the Institute of Food Technologists, New Orleans, La., June, 23-27, 2001. The author would like to acknowledge the contributions of all of his 46 graduate students who contributed significantly to whatever success he has had in his career. He trusts that they learned as much from him as he learned from each of them.
by Edmund A. Zottola
The author, a Fellow and Professional Member of IFT and Professor Emeritus of the University of Minnesota’s Dept. of Food Science and Nutrition, is at 2866 Vermilion Ave., Cook, MN 55723.
Edited by Neil H. Mermelstein, Editor
References
Anonymous. 1994. Biofilm heterogeneity. Biofilm Engineering News 2(1): xx. Center for Biofilm Engineering, Montana State Univ., Bozeman.
CDC. 1982. Epidemiological notes and reports. Isolation of Escherichia coli O157:57 from sporadic cases of hemorrhagic colitis—United States. Center for Disease Control, Morbidity Mortality Weekly Rept. 31: 580-585
CDC. 1998. Multistate outbreak of Salmonella serotype agona infections linked to toasted oats cereal—United States, April-May, 1998. Center for Disease Control, Morbidity Mortality Weekly Rept. 44: 462-464.
CDC. 1993. Update: Multi-state outbreak of Escherchia coli O157:H7 infections from hamburgers—Western United States. Center for Disease Control, Morbidity Mortality Weekly Rept. 42:258-263.
Characklis, W.G. and Cooksey, K.E. 1983. Biofilms and Microbial fouling. Adv. Appl. Microbiol. 29: 93-137.
Collins, R.N., Treger, M.D., Goldsby, J.B., and Coohon, D.B. 1966. Interstate outbreak of Salmonella newbrunswick infection traced to powdered milk. I. Development of epidemiological hypothesis. Presented at Ann. Mtg., Am. Public Health Assn.
Hennessey, T.W., Hedberg, C.W., Slutsker, L., White, J. M., Moen, M.E. 1996. A national outbreak of Salmonella enteritidis infections from ice cream. New Eng. J. Med. 364: 1281-1286.
Herald, P.J. and Zottola, E.A. 1988. Scanning electron microscopic examination of Yersinia enterocolitica attached to stainless steel at selected temperatures and pH levels. J. Food Protect. 51: 445-448.
Hood, S.K. and Zottola, E.A. 1995. Biofilms in food processing. Food Control. 6(1): 9-18.
Lovett, J. S. 1989. Listeria monocytogenes.. Chpt. 7 in “Foodborne Bacterial Pathogens,” ed. M.P Doyle, pp. 283-306. Marcel Dekker, Inc., New York.
Marshall, K.C. 1992. Biofilms: An overview of bacterial adhesion, activity and control at surfaces. ASM News 58: 202-207.
Marshall, K.C., Stout, R., and Mitchell, R. 1971. Mechanisms of Initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 68: 337-348.
NAS. 1969. “An Evaluation of the Salmonella Problem.” Natl. Academy of Sciences, Washington, D.C.
Petran, R. and Zottola, E.A. 1988. Survival of Listeria monocytogenes in simulated milk cooling Systems. J. Food Protect. 51: 172-175.
Pillsbury. 1973. Food safety through the Hazard Analysis and Critical Control Point system. Pillsbury Co., Minneapolis.
Riley, L.W., Remis, R.S., and Helgerson, S.D. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New Eng. J. Med. 308: 691-685.
Ryan, C.A., Nickels, M.K., Hagrett-Bean, N.T., Potter, M.E., Endo, T., Mayer, L., Langkop. C.W., Gibson, C., MacDonald, R.C., Kenny, R.T., Puhr, N.D., McDonnell, P.J., Martin,M.L., Cohen, M.L., and Blake, P.A. 1987. Massive outbreak of antimicrobial-resistant salmonellosis traced to pasteurized milk. J. Am. Med. Assn. 258: 3269-3274.
Sanders, E.E., Friedman, A., Boring, J.R. III, Hanlon, J.J., Polk, L.D., Sweeney, F.J. and Randall, E.L. 1965. Interstate outbreak of Salmonella derby gastroenteritis. Proc. of Natl. Conf. on Salmonellosis. Public Health Service Pub. 1262. U.S. Dept. of Health, Education and Welfare, Washington, D.C.
Sasahara, K.C. and Zottola, E.A. 1993. Biofilm Formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J. Food Protect. 56: 1022-1028.
Schuman, J.D., Zottola, E.A., and Harlander, S. K. 1989. Preliminary characterization of a food-borne multiple-antibiotic-resistant Salmonella typhimurium strain. Appl. Environ. Microbiol. 55:9: 2344-2348.
Schwach, T.S. and Zottola, E.A. 1982. Use of scanning electron microscopy to demonstrate microbial attachment to beef and beef contact surfaces. J. Food Sci. 47: 1401-1405.
Schwach, T.S. and Zottola, E.A. 1984. Scanning electron microscopic study of some effects of sodium hypochlorite on attachment of bacteria to stainless steel. J. Food Protect. 47: 756.
Zottola, E.A. 1976. Botulism. Bull. 372. Minnesota Extension Service, Univ. of Minnesota, St. Paul.
Zottola, E.A. 1997. Special techniques for studying microbial biofilms in food systems. In “Food Microbiological Analysis—New Technologies,” ed. M.L. Tortorello and S.M. Gendel. Marcel Dekker, New York.
