Neotame: The Next-Generation Sweetener
A new sweetener derived from aspartame is thousands of times sweeter than sugar and does not have the undesirable taste characteristics common to some high-intensity sweeteners.
Neotame, a new high-intensity sweetener and flavor enhancer, is expected to receive Food and Drug Administration approval for use in foods and beverages in the United States soon. Since it will then be the newest approved sweetener in the U.S., it is appropriate to review its development, characteristics, and potential uses.
Overview
Neotame is a derivative of the dipeptide composed of the amino acids aspartic acid and phenylalanine. It is 7,000–13,000 times as sweet as sugar and 30–60 times as sweet as aspartame. It is manufactured by The NutraSweet Co., Mt. Prospect, Ill., the company that developed the noncaloric sweetener aspartame.
It provides zero calories and has a clean, sweet, sugar-like taste with no undesirable taste characteristics. It is functional in a wide array of beverages and foods and can be used alone or blended with other high-intensity or carbohydrate sweeteners. It is stable under dry conditions, and has comparable stability to aspartame in aqueous food systems and more stable in neutral pH conditions (e.g., baking and yogurt).
The results of numerous safety studies confirm that it is safe for use by the general population, including children, pregnant women, and people with diabetes. In addition, since the product is not metabolized to phenylalanine, no special labeling for individuals with phenylketonuria (PKU) is required. Neotame has been approved for general use as a sweetener and flavor enhancer in Australia and New Zealand and is being reviewed in the U.S. and other countries.
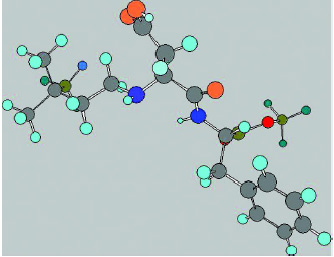 Discovery and Manufacture
Discovery and Manufacture
Neotame was the result of a long-term research program by The NutraSweet Co. to discover new high-intensity sweeteners with desirable taste characteristics. Working with The NutraSweet Co., French scientists Claude Nofre and Jean-Marie Tinti prepared a series of compounds by substituting the terminal nitrogen of aspartame with a number of hydrophobic groups and determined their sweetness compared to a 2% solution of sucrose. Aspartame substituted with a 3,3-dimethylbutyl group was the sweetest of the compounds tested and was selected as development product and called neotame (Nofre and Tinti, 1996b, 2000). This compound has the chemical structure as shown in Fig. 1.
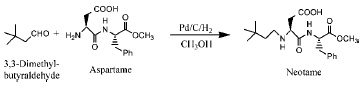 As shown in Fig. 2, neotame can be made in one step by the reaction of aspartame with 3,3-dimethylbutyraldehyde in methanol, using hydrogen and a catalyst (palladium or platinum) under mild conditions (Nofre and Tinti, 1996a; Prakash, 1998). Other possible methods of preparation are from aspartame precursors via the reductive alkylation with 3,3-dimethylbutyraldehyde; peptide coupling of the L-aspartic acid derivatives and L-phenylalanine methyl ester; aminolysis of substituted oxazolidinone derivatives (Prakash, 2001; Prakash and Chapeau, 2000; Prakash et al., 2001b).
As shown in Fig. 2, neotame can be made in one step by the reaction of aspartame with 3,3-dimethylbutyraldehyde in methanol, using hydrogen and a catalyst (palladium or platinum) under mild conditions (Nofre and Tinti, 1996a; Prakash, 1998). Other possible methods of preparation are from aspartame precursors via the reductive alkylation with 3,3-dimethylbutyraldehyde; peptide coupling of the L-aspartic acid derivatives and L-phenylalanine methyl ester; aminolysis of substituted oxazolidinone derivatives (Prakash, 2001; Prakash and Chapeau, 2000; Prakash et al., 2001b).
Characteristics
Neotame’s physical, chemical, and sensory characteristics make it attractive for use as a sweetener in foods and beverages.
--- PAGE BREAK ---
• Chemical Characteristics. Neotame is N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester (CAS registry No. 165450-17-9, proposed INS No. 961). It is a derivative of a dipeptide composed of the amino acids aspartic acid and phenylalanine. It contains both a carboxylic acid and a secondary amino group, with pKa values of 3.03 and 8.08, respectively. It is capable of forming both acidic and basic salts, as well as complexes with various metals, thus affording unique delivery forms having improved solubility and other characteristics.
The two amino acids in neotame, aspartic acid and phenylalanine, are in the natural L-configuration. The other three possible isomers, L,D-, D,D-, and D,L-, lack the sweet taste of neotame (Prakash et al., 1999).
• Physical Characteristics. Neotame is a fairly low-melting hydrate (80.9–83.40C). It is a white to off-white crystalline powder with 4.5% water of hydration, the empirical formula C20H30N2O5 • H2O, and a molecular weight of 396.48.
Its solubility in water is similar to that of aspartame (12.6 g/L vs 10 g/L at 250C), but it is more readily soluble than aspartame in some solvents, such as ethanol, typically used in food systems and pharmaceuticals. Its solubility in water and ethyl acetate increases with increasing temperature. Using neotame in a salt form (e.g., as a phosphate salt) significantly increases the rate of dissolution.
• Stability. The stability of neotame is dependent on pH, moisture, and temperature. As a dry powder, it is stable for at least five years under proper storage conditions. In aqueous systems, pH stability follows a bell-shaped curve at a given temperature. The optimum pH for maximum stability is about 4.5. As expected, stability decreases with increasing temperature. Stability can be enhanced by the addition of divalent or trivalent cations in edible compounds (Schroeder and Wang, 2001b).
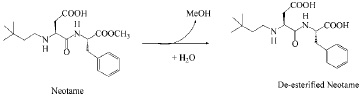 In aqueous systems (pH 2–8), the major decomposition pathway of neotame is through the hydrolysis of the methyl ester to form de-esterified or de-methylated neotame—N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine (Fig. 3)—which is also the major metabolite of neotame in humans. Deesterified neotame is not sweet.
In aqueous systems (pH 2–8), the major decomposition pathway of neotame is through the hydrolysis of the methyl ester to form de-esterified or de-methylated neotame—N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine (Fig. 3)—which is also the major metabolite of neotame in humans. Deesterified neotame is not sweet.
Under conditions of use, neotame, unlike aspartame, does not degrade to phenylalanine. Also unlike aspartame, neotame does not form a diketopiperazine (DKP) derivative. Neotame is compatible with reducing sugars and aldehyde or ketone-based flavoring agents.
• Sweetness. Sucrose is the sweetness standard against which other compounds are compared. A compound with a “sucrose equivalence” of x% SE is equivalent in sweetness to an x% solution of sucrose in water. Neotame is approximately 8,000 times as sweet as sucrose and more potent than the high-intensity sweeteners currently marketed in the U.S.—aspartame and acesulfame K (200 times as sweet as sucrose), saccharin (300 times), and sucralose (600 times). It is a derivative of aspartame and is 30–60 times sweeter than aspartame. Its actual sweetness potency is dependent on the concentration required in various food or beverage products.
Because of its remarkable sweetness potency, neotame can be used in food and beverage products at considerably lower concentrations than other high-intensity sweeteners. In fact, consumer exposure to neotame will be much lower than exposure to flavoring ingredients such as vanillin, cinnamon, and menthol commonly used in foods and beverages.
--- PAGE BREAK ---
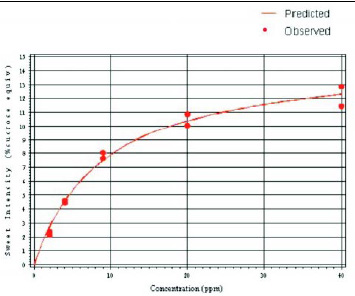 The concentration–response curve for neotame (Fig. 4) was established using a trained sensory panel to evaluate the sweetness intensity of five solutions of neotame at increasing concentrations. Based on these data, neotame can reach an extrapolated maximum sweetness intensity (plateau) of 15.1% SE in water. Sweeteners such as aspartame, acesulfame K, sodium cyclamate, and sodium saccharin attain their maximum sweetness intensity in water at approximately 16.0, 11.6, 11.3, and 9.0% SE, respectively. In a cola formulation, neotame reaches a maximum sweetness intensity of 13.4% SE (DuBois et al., 1991).
The concentration–response curve for neotame (Fig. 4) was established using a trained sensory panel to evaluate the sweetness intensity of five solutions of neotame at increasing concentrations. Based on these data, neotame can reach an extrapolated maximum sweetness intensity (plateau) of 15.1% SE in water. Sweeteners such as aspartame, acesulfame K, sodium cyclamate, and sodium saccharin attain their maximum sweetness intensity in water at approximately 16.0, 11.6, 11.3, and 9.0% SE, respectively. In a cola formulation, neotame reaches a maximum sweetness intensity of 13.4% SE (DuBois et al., 1991).
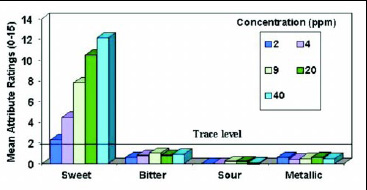
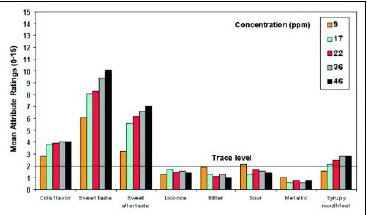 • Taste Profile. A trained descriptive panel evaluated neotame and sucrose at comparable sweetness levels in water. Neotame’s taste profile is very similar to that of sucrose, with the predominant sensory characteristic being a very clean, sweet taste. The sweetness increases as the concentration in water increases, but other taste attributes such as bitterness, sourness, and metallic taste are insignificant (Fig. 5). In a similar study with neotame in a cola drink, increasing the sweetener concentration from 9 to 46 ppm improved the desirable flavor attributes (cola flavor, sweet taste, and mouthfeel) but did not increase the undesirable notes (Fig.6).
• Taste Profile. A trained descriptive panel evaluated neotame and sucrose at comparable sweetness levels in water. Neotame’s taste profile is very similar to that of sucrose, with the predominant sensory characteristic being a very clean, sweet taste. The sweetness increases as the concentration in water increases, but other taste attributes such as bitterness, sourness, and metallic taste are insignificant (Fig. 5). In a similar study with neotame in a cola drink, increasing the sweetener concentration from 9 to 46 ppm improved the desirable flavor attributes (cola flavor, sweet taste, and mouthfeel) but did not increase the undesirable notes (Fig.6).
• Sweetness Temporal Profile. The temporal profile of sweeteners demonstrates the changes in the perception of sweetness over time. This property is a key to the functionality of a sweetener and is complementary to its taste profile. Every sweetener exhibits a characteristic onset or response time and an extinction time. Most high-intensity sweeteners, in contrast to sugar, display a prolonged extinction time referred to as “linger.”
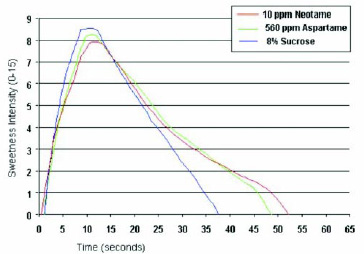 As shown in Fig. 7, the sweetness temporal profile of neotame in water is close to that of aspartame, with a slightly slower onset and slightly longer linger. A longer sweetness linger can be beneficial in some products, such as chewing gum, where prolonged sweetness is a desirable quality.
As shown in Fig. 7, the sweetness temporal profile of neotame in water is close to that of aspartame, with a slightly slower onset and slightly longer linger. A longer sweetness linger can be beneficial in some products, such as chewing gum, where prolonged sweetness is a desirable quality.
The sweetness temporal profile of neotame may also be modified by the addition of hydrophobic organic acids, such as cinnamic acid, and certain amino acids, such as serine and tyrosine (Bishay et al., 2000b; Gerlat et al., 2000; Prakash et al, 2001a). Taste modifiers may be used in concentrations necessary to achieve the desired taste profile of a product for a desired application.
--- PAGE BREAK ---
• Synergy. Blending of sweeteners is well known to improve taste characteristics and stability and provide sweetness synergy (Lavia and Hill, 1972; Schiffman et al., 1995; Scott, 1971; Verdi and Hood, 1993; Walters, 1993). A blend of neotame and saccharin provides 14–24% greater sweetness than would be predicted by adding together the sweetness intensities of the individual sweeteners (Pajor and Gibes, 2000). Such synergistic blends offer cost savings by decreasing the total amount of sweetener needed. Neotame can be blended with nutritive sweeteners as well as other high-intensity sweeteners such as aspartame, acesulfame salts, cyclamate, sucralose, saccharin, and others (Nofre and Tinti, 1996b). Furthermore, the clean sweetness of neotame permits its substitution for substantial amounts of carbohydrate sweeteners without altering the flavor of the product.
Because time–intensity profiles of the sweeteners acting synergistically are different from those of the individual sweeteners and may also be different from that of sucrose, blends can be selected that combine or emphasize properties of the different sweeteners. The sweetness of acesulfame K is generally perceived fairly quickly. It may, therefore, provide some impact sweetness, but it often fades fairly quickly. Therefore, acesulfame combines particularly well with sweeteners having a more lasting sweetness, such as aspartame or neotame.
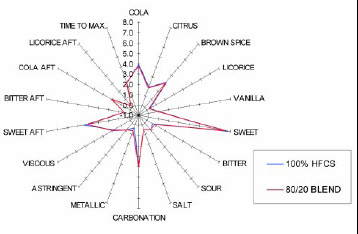 • Sugar Substitution. Neotame’s clean sweet taste allows the food technologist to substitute a portion of a carbohydrate sweetener with neotame while maintaining a taste that is indistinguishable from the 100% carbohydrate product. For example, studies have shown that 20% of the carbohydrate sweetener can be replaced with 2.1 ppm of neotame in a carbonated cola soft drink, and the taste is indistinguishable from the 100% carbohydrate–sweetened cola beverage (Fig. 8). Neotame’s potency may offer an economic benefit and, because it has no calories, a positive caloric benefit.
• Sugar Substitution. Neotame’s clean sweet taste allows the food technologist to substitute a portion of a carbohydrate sweetener with neotame while maintaining a taste that is indistinguishable from the 100% carbohydrate product. For example, studies have shown that 20% of the carbohydrate sweetener can be replaced with 2.1 ppm of neotame in a carbonated cola soft drink, and the taste is indistinguishable from the 100% carbohydrate–sweetened cola beverage (Fig. 8). Neotame’s potency may offer an economic benefit and, because it has no calories, a positive caloric benefit.
• Flavor Modification and Enhancement. Neotame can also be used to modify or enhance a product’s flavor—the combined perception of taste, smell, and aroma. Products containing vitamins, nutraceuticals, pharmaceuticals, salt substitutes, and soy in various applications are often either bitter or harsh in flavor. The addition of neotame at a subsweetening level modifies or masks undesirable notes/qualities such as bitterness, astringency, and burning or cooling sensations. Undesirable attributes, such as the potential bitterness of caffeine, cocoa, and potassium chloride and the harsh notes of medicinals and plant extracts, can be modified or masked.
Neotame also reduces the bitter taste of potassium chloride in salt substitutes, thereby providing a cleaner salty taste. It reduces or eliminates “beany” flavor notes in soy products. And it modifies or enhances the attributes of many flavoring chemicals, including essential oils, oleoresins, plant extracts, reaction flavors, and mixtures thereof (Gerlat et al., 2000).
Food Applications
Historically, the stability and functionality of a new sweetener or an ingredient was determined for each food product before the sweetener was approved. This process generated redundant data. This redundancy could be avoided if products with similar ingredients and processing conditions could be reduced to representative test products for evaluation.
--- PAGE BREAK ---
The functionality of neotame was demonstrated with a three-dimensional food matrix model representing the intended conditions of use in foods (Pariza et al., 1998). Based on experience with aspartame and the structural similarities of neotame and aspartame, product moisture, process temperature, and product pH were considered to be the key factors responsible for neotame stability and were selected to represent the three dimensions of the matrix.
Test products were prepared according to standard formulas, then packaged appropriately, stored at temperature conditions of up to 25ºC and 60% relative humidity, and evaluated for stability at appropriate intervals. Neotame concentrations were determined using validated high-performance liquid chromatography methods.
Functionality (sweetness) of the test products was determined using panels consisting of 35–50 persons. Samples were appropriately prepared, served, and evaluated on a scale ranging from 5 (“much too sweet”) to 1 (“not at all sweet”). The samples were considered functional if no more than 75% of the panelists rated the sweetness as 2 (“not quite sweet enough”) and 1.
• Cola-Flavored Carbonated Soft Drink. Neotame remained functional for at least 16 weeks, consistent with currently marketed low-calorie carbonated soft drinks (Gerlat et al., 1999).
• Hot-Pack Lemon Tea. Neotame remained functional for approximately 25 weeks.
• Powdered Soft Drink. At each evaluation, the sweetness of the reconstituted drink received a rating of “just about right,” indicating that the product was stable and functional as a sweetener during 52 weeks of storage.
• Tabletop Products. Neotame was considered stable and functional in tabletop products for at least 156 weeks of storage (FAP, 1998; Towb et al., 2002).
• Chewing Gum. Encapsulation improved neotame stability. Double coating with modified starch and hydroxypropyl methylcellulose protected it from degradation during storage for 52 weeks (Roefer, 2002).
• Dairy Products/Strawberry Yogurt. At the end of a 6-week period, the typical shelf life of these products, about 98% of the initial neotame remained. Sensory results showed that neotame had excellent functionality in yogurt (FAP, 1998; Gaughan et al., 1999).
• Yellow Cake. Neotame was functional, with 82% of the amount added to the batter remaining after baking at 350ºF. After storage at 25ºC and 60% relative humidity for 5 days—which is longer than cakes baked from commercial mixes are held for optimum freshness—there was only a 4% loss of neotame. The combined losses of about 20% did not affect sweetener functionality (Chinn et al., 1999; FAP, 1998).
• Other Products. Functionality has also been demonstrated in cereals and cereal-based foods (Ponakala and Corliss, 2000), nutraceuticals (Ponakala et al., 2000a), pharmaceuticals (Ponakala, 2001), edible gels (Ponakala et al., 2000b), and confectionery products (Jarrett, 2001).
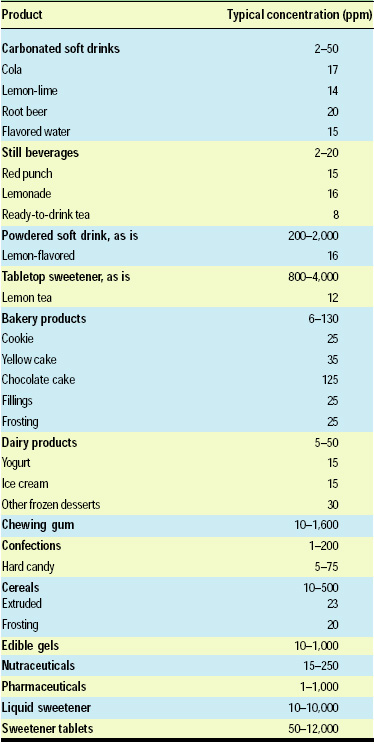 Table 1 presents some typical use levels of neotame in various foods and beverages. Since neotame is extremely sweet, the use levels are expressed as parts per million rather than percentages. The ranges provided are for neotame used either as a single sweetener or as a component in a sweetener blend.
Table 1 presents some typical use levels of neotame in various foods and beverages. Since neotame is extremely sweet, the use levels are expressed as parts per million rather than percentages. The ranges provided are for neotame used either as a single sweetener or as a component in a sweetener blend.
--- PAGE BREAK ---
Delivery Forms and Benefits
Neotame can be prepared in a wide variety of forms, including agglomerated (Fotos and Bishay, 2001), granulated (Dron, 2001), extruded and spheronized (Dron et al, 2000), encapsulated (Ponakala et al., 1999), co-crystallized with sugar (Fotos et al., 2001), acid salts (Prakash and Wachholder, 2001a), basic salts (Prakash and Wachholder, 2001b), sweetener salts (Prakash and Guo, 2000b), amorphous (Schroeder and Wang, 2001a), metal complexes (Prakash and Guo, 2000a), cyclodextrin complexes (Bishay et al., 2000a), and liquid (Schroeder et al, 2000). In certain uses, these delivery forms offer various advantages over neotame powder, such as ease of handling, non-dustiness, and improved solubility characteristics, bringing greater flexibility to product developers.
Neotame provides several benefits as a sweetener and/or flavor enhancer in food and beverage systems. It is noncaloric; it requires no PKU labeling; it is not likely to react with aldehydes and consequently may be compatible with flavors containing aldehydes. Because of its high potency, the quantity required to sweeten a product is about 1/30 to 1/60 of the amount of aspartame required. It enhances the flavor of some ingredients, such as mint and suppresses the beany notes of soy, in various food and beverage systems. It masks bitterness. It can complement the flavor of root beer beverages. In fruit-based juices, because of the increased mouthfeel it contributes, juice solids can be reduced. It has a sparing effect on the flavoring agent vanillin in puddings; on cocoa, dairy component, and vanillin in chocolate and cocoa-based products; on dairy and fruit components, such as citric acid, in yogurt; and on tomato flavor in barbeque sauces.
Safety and Regulatory Status
The results of extensive research done in animals and humans using amounts of neotame that far exceed expected consumption levels clearly confirm its safety for the general population, including children, pregnant women, and people with diabetes. Neotame is not mutagenic, teratogenic, or carcinogenic and has no effect on reproduction. In addition, no special labeling for phenylketonuric individuals is required. The major route of metabolism of neotame is de-esterification. Both neotame and de-esterified neotame have short plasma half-lives, with rapid and complete elimination (FAP, 1998, 1999).
The Food and Drug Administration is currently reviewing a food additive petition for approval of neotame for general use in food as a sweetener and flavor enhancer, and petitions for regulatory approval have been filed in a number of foreign countries. Australia and New Zealand have already approved use of neotame as a sweetener and flavor enhancer.
Neotame’s unique properties will provide the food technologist with another tool to produce innovative new foods and beverages to meet consumers’ demands for great-tasting foods without all the calories of sugar.
by Indra Prakash, Glenn Corliss, Rao Ponakala, and Glen Ishikawa
The authors are, respectively, Director of Organic Chemistry, Senior Food Scientist, Senior Food Scientist, and Director of R&D, The NutraSweet Co., 699 Wheeling Rd., Mt. Prospect, IL 60056. Authors Corliss, Ponakala, and Ishikawa are Professional Members of IFT. Send reprint requests to author Prakash.
References
Bishay, I., Fotos, J., Desai, N., Cleary, M., and Schroeder, S. 2000a. The use of cyclodextrin to stabilize N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. PCT intl. applic. WO 00/15049.
Bishay, I., Prakash, I., Desai, N., and Gelman, Y. 2000b. Modification of the taste and physicochemical properties of neotame using hydrophobic additives. PCT intl. applic. WO 00/69282.
Chinn, B., Solter, S., Ponakala, S., Ziegler, J., Bakhoum, M., Jarrett, T., Paeschke, T., and Corliss, G. 1999. Use of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester in baked goods, frostings and bakery fillings. PCT intl. applic. WO 99/30566.
Dron, A. 2001. Process for making granulated N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-Lphenylalanine 1-methyl ester. PCT intl. applic. WO 01/60842.
Dron, A., Bishay, I., Fotos, J., and Trione, M. 2000. Spheronization of neotame with and without binders. PCT intl. applic. WO 00/57725.
DuBois, G., Walters, D, Schiffman, S., Warwick, Z., Booth, B., Pecore, S., Gibes, K., Carr, B., and Brands, L. 1991. Concentration-response relationships of sweeteners. Chpt. 20 in “Sweeteners Discovery, Molecular Design, and Chemoreception,” ed. D.E. Walters, F.T. Orthoefer, and G.E. DuBois, pp. 261-276. Am. Chem. Soc., Washington, D.C.
FDA. 1998. Monsanto Co.: Filing a food additive petition. FAP 8A4580. Food and Drug Admin., Fed. Reg. 63: 6762.
FDA. 1999. Monsanto Co.: Filing a food additive petition. FAP 9A4643. Food and Drug Admin., Fed. Reg. 64: 6100.
Fotos, J. and Bishay, I. 2001. Process for preparing an N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester agglomerate. U.S. patent 6,180,157.
Fotos, J., Bishay, I., Prakash, I., Wachholder, K., and Desai, N. 2001. Co-crystallization of sugar and N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,214,402.
Gaughan, W., Gerlat, P., Ziegler, J., Walters, G., Logli, L., Corliss, G., and Finley, J. 1999. Neotame sweetener for dairy products and dairy product substitutes. PCT intl. applic. WO 99/30578.
Gerlat, P., Hatchwell, L., Walters, G., Miragilo, A., and Sawer, H. 2000. Use of N-neohexyl-α-aspartyl-L-phenylalanine methyl ester as flavor modifier. U.S. patent applic. 09/465,837.
Gerlat, P., Milovanovic, S., Ponakala, S., Ziegler, J., Sawyer, H., and Walters, G. 1999. Neotame-based sweeteners for beverages. PCT intl. applic. WO 99/30576.
Gerlat, P., Walters, G., Bishay, I., Prakash, I., Jarrett, T., Desai, N., Sawer, H., and Bechert, C.-L. 2000. Use of additives to modify the taste characteristics of N-neohexyl-α-aspartyl-L-phenylalanine methyl ester. PCT intl. applic. WO 00/69283.
Jarrett, T. 2001. Confectionery food products sweetened with N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. PCT intl. applic. WO 01/010236.
Lavia, A. and Hill, J. 1972. Sweeteners with masked saccharin aftertaste. French patent 2,087,843.
Nofre, C. and Tinti, J.-M. 1996a. Method of preparing a compound derived from aspartame, useful as a sweetening agent. U.S. patent 5,510,508.
Nofre, C. and Tinti, J.-M. 1996b. N-substituted derivatives of aspartame useful as sweetening agents. U.S. patent 5,480,668.
Nofre, C. and Tinti, J.-M. 2000. Neotame: Discovery, properties, utility. Food Chem. 69: 245-297
Pajor, L. and Gibes, K. 2000. N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester synergistic sweetener blends. U.S. patent 6,048,999.
Pariza, M., Ponakala, S., Gerlat, P., and Andress, S. 1998. Predicting the functionality of direct food additives. Food Technol. 52(11): 56-60.
Ponakala, S. 2001. Pharmaceutical compositions containing neotame. PCT intl. applic. WO 01/028590.
Ponakala, S. and Corliss, G. 2000. Cereals and cereal-based food sweetened with neotame. PCT intl. applic. WO 00/056175.
Ponakala, S., Ziegler, J., Patterson, K., Brahmbhatt, D., Lui, P., Corliss, G., Chaudhary, V., Towb, A., Bishay, I., and Ansay, A., R. 1999. N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester as a sweetener in chewing gum. U.S. patent applic. No. 09/465,402.
Ponakala, S., Walters, G., Gerlat, P., and Hatchwell, L. 2000a. Nutraceuticals having N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. PCT intl. applic. WO 00/057726.
Ponakala, S., Walters, G., and Schroeder, S. 2000b. Manufacture of edible gels sweetened with neotame. PCT intl. applic. WO 00/056176.
Prakash, I. 1998. Method for preparing and purifying an N-alkylated aspartame derivative. U.S. patent 5,728,862.
Prakash, I. 2001. Synthesis of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester using oxazolidinone derivatives. PCT intl. applic. WO 01/90138.
Prakash, I. and Chapeau, M.-C. 2000. N-3,3-Dimethylbutyl-L-aspartic acid and esters thereof, the process of preparing the same, and the process for preparing N-(N-(3,3-dimethylbutyl)- L-α-aspartyl)-L-phenylalanine 1-methyl ester therefrom. U.S. patent 6,077,962.
Prakash, I., and Guo, Z., 2000a. Metal complexes of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,146,680.
Prakash, I., and Guo, Z., 2000b. Sweetener salts of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,129,942.
Prakash, I., and Wachholder, K. 2001a. Acid salts of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,180,156.
Prakash, I. and Wachholder, K. 2001b. Basic salts of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,291,004.
Prakash, I., Bishay, I., and Schroeder, S. 1999. Neotame: synthesis, stereochemistry and sweetness. Synthetic Commun. 29: 4461-4467.
Prakash, I., Bishay, I., Desai, N., and Walters, D. 2001a. Modifying the temporal profile of the high-potency sweetener neotame. J. Agric. Food Chem. 49: 786-78.
Prakash, I., Scaros, M., Orlovskii, V., and Moore, C. 2001b. A method for the preparation of N-neohexyl-L-α-aspartyl-L-phenylalanine methyl ester from imidazolidin-4-one intermediates. PCT intl. applic. WO 01/87927.
Roefer, W. 2002. Unpublished data. Dept. of Analytical Chemistry, The NutraSweet Co., Mt. Prospect, Ill.
Schiffman, S., Booth, B., Carr, B., Losee, M., Sattely-Miller, E., and Graham, B. 1995. Investigation of synergism in binary mixtures of sweeteners. Brain Research Bull. 38(2): 105-120.
Schroeder, S. and Wang, R. 2001a. Amorphous N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester. U.S. patent 6,331,646.
Schroeder, S. and Wang, R. 2001b. Stability enhancement of sweeteners using salts containing divalent or trivalent cations. PCT intl. applic. WO 01/70049.
Schroeder, S., Wang, R., Ponakala, S., and Choudhary, V. 2000. Method of preparing liquid compositions for delivery of N-[N-(3,3-dimethylbutyl)-L-α-aspartyl]-L-phenylalanine 1-methyl ester in food and beverage systems. PCT intl. applic. WO 02/05661.
Scott, D. 1971. Saccharin-dipeptide sweetening compositions. British patent 1,256,995.
Towb, A., Dron, A., and Walters, G. 2002. Drying of neotame with co-agents. PCT intl. applic. WO 02/05660.
Verdi, R.J. and Hood, L.L. 1993. Advantages of alternative sweetener blends. Food Technol. 47(6): 94-101.
Walters, E. 1993. High intensity sweetener blends. Food Prod. Design 3(6): 83-92.
