Recreating Flavors from Nature
Chiral chemistry and advanced technologies produce natural flavors with key nuances and artificial flavors that duplicate the taste and aroma of natural flavors.
The creation of flavors has always involved both the artistry of the flavorist and the science of the chemist. Creation of a flavor that smells and tastes close to nature has been an ongoing challenge for decades. Flavorists strive to imitate nature in creating flavors that mimic the fresh, juicy, green, and ripe aroma and taste of the original food, spice, or herb. In other words, the goal of the flavorist is to create those subtle flavor nuances that excite our senses of taste and smell.

In the past, creation of “natural-like” flavors was not possible because of various technical challenges. These included the lack of sophisticated analytical instruments and techniques that could identify the true drivers needed to help flavorists recreate nature. The dilemma flavorists often faced was that their noses could identify aromas but the instrumentation was not capable of identifying those chemicals accurately. This prevented flavorists from identifying those key ingredients and synthesizing the molecules that occur in nature.
The challenge was to imitate nature in all its glory, and flavorists often succumbed to the practice of introducing too many chemicals in the creation of a flavor just to capture that one elusive note. The result was analogous to colors—when an artist blends too many colors to create a secondary color, the resulting painting loses its vibrancy and hue. Similarly, when chemicals which are not inherent and true in nature are introduced, the resulting flavor loses its nuances and intensity.
Creating a New Paradigm
The journey that began decades ago is at last winding down. Many of nature’s secrets have been deciphered. Analytical chemistry has yielded enough qualitative and quantitative information to synthesize new molecules. The work that lies ahead involves identifying and separating the specific compounds responsible for the characteristic notes found in flavors derived from nature, namely, the chiral aroma compounds, discussed below.
Many of the specific chiral components have been found to be of great interest from a sensory viewpoint and useful in adding the nuances needed to create improved flavors. For example, certain of the fruity volatile chiral esters have a cleaner aroma and taste than the racemic mixtures. Although these findings have been useful, the commercial success of these materials requires significant work by scientists to develop methods of synthesizing or capturing from nature these specific components.
The complexity of nature also adds to the overall sensory properties of the flavor and includes mouthfeel and taste sensations that come from natural components such as flavonoids, tannins, and precursors. Hence, for a total reconstitution of a nature-like flavor, it is necessary to include some of these ingredients in a flavor formulation. However, synthesizing or isolating them from natural sources can be complex, at times technically difficult, and costly. One option is to incorporate ingredients isolated from natural juices, extracts, essences, or distillates. Recent advances in extraction technologies provide us with ingredients that are superior to prior marketed ingredients for use in creating flavors that are closer to the profiles of the flavor found in the natural foods, herbs, and spices. The change in the flavor paradigm requires that many of these technologies be integrated to create the final superior flavor.
Identifying Key Components
The isolation and separation of natural aromas from food can be achieved by several different techniques and procedures. Success depends on choosing the technique that will give the information needed in the time frame available. In the flavor business, choosing the proper natural aroma for the flavor systems being designed is often the difference between winning and losing “the business” from our customers.
First, the key components of a natural flavor—the chiral aroma chemicals—must be identified by chemical analysis of natural products. We use our patented Aromashuttle®, a portable and easy-to-use odor-sampling method that allows the trapping and identification of the natural aromas from living plants in the fields and hot houses where they grow (Chinn and Nakatsu,1999). It also allows for the trapping of the volatile complexes during the growth and maturity of the fruit.
--- PAGE BREAK ---
In this method, an adsorbent coating is affixed to the internal wall of a sealable container (Fig. 1). The container may be rigid or flexible. When the container is sealed, the adsorbent coating retains odor components for an extended period, permitting them to be transported without requiring the transport of the source of the components. Thermal or other desorption techniques release the components for analysis by gas chromatography–mass spectroscopy (GC-MS), as described below, or smelling the aroma.
Once the natural aroma from the food material has been isolated, it is critical to obtain a good separation of the components or chemicals that constitute the aroma. Gas chromatography has classically been used to separate the individual components, and mass spectrometry has been used to identify each component. An example of the power of this GC-MS technique coupled with our proprietary static headspace sampling method (Crownspace®) using the Aro-mashuttle technology, can be seen in Fig. 2, where the volatile profile of honey from different sources can be differentiated by their resulting GC profiles. The information obtained from the GC gives the intensities of the volatile compounds, and the GC-MS analysis identifies them. Knowledge of the volatile components is important, but they may not play a significant role in the overall aroma of the material being investigated. We use another technique called gas chromatography olfactometry (GCO) to identify the aroma component found in the trapped mixture of volatiles. The GCO techniques will be discussed later in this article.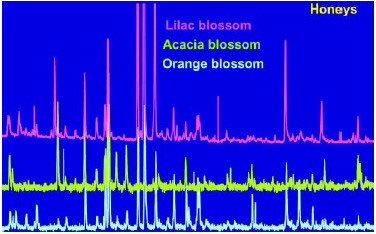
Specialized flavor and fragrance mass spectral libraries are used to help identify each component. Special GC detectors can be used to obtain increased sensitivity to certain components, such as those containing sulfur and nitrogen. A GC coupled with an atomic emission detector (GC-AED), for example, can be used to detect trace concentrations of components containing sulfur, nitrogen, or phosphorus. Many of these components have very low odor thresholds and significantly contribute to both the characterizing aroma and nuances of the original aroma.
In some cases, the most important components of the natural aroma are below the MS detection capability. The human nose is a much more sensitive detector of volatiles that have aroma. In many cases, the components in an aroma profile produced by GC may not be those with significant aroma; however, the trace components that escape the MS or AED detection can be found by GCO analysis. In this technique, flavorists and analytical chemists prepare various extracts of natural products and inject them into a GC, then sniff and characterize the compounds as they elute from the GC (Fig. 3).
Then, in a technique called aroma extract dilution analysis (AEDA), samples are diluted stepwise until the trained flavorist can no longer detect the odor of any aroma compounds. The advantage of this method is that the “key drivers of aroma” can be identified and resolved from the GC chromatogram.
The aroma compounds are assigned a flavor dilution (FD) factor. The higher the FD factor, the greater the aroma strength of the individual compounds. For example, if an aroma compound was last detected after three successive dilutions, that compound would receive an FD value of 3. From these data, an aromagram (Fig. 4) is created, showing the difference when compared to a standard GC chromatogram (dotted lines in the GC chromatogram show where strong aroma compounds are found in the aromagram). Often, a GC chromatogram and an aromagram look very different. This is because some strong aroma compounds are often not detected by GC but are detected by the human nose. This information is extremely important to the flavorist for formulation of a flavor to simulate the natural product because these compounds would often go undetected using GC or GC-MS only. The retention times of these trace odor compounds, or key drivers of aroma, are noted, and the samples are concentrated and reinjected at the same exact conditions into a GC-MS for identification.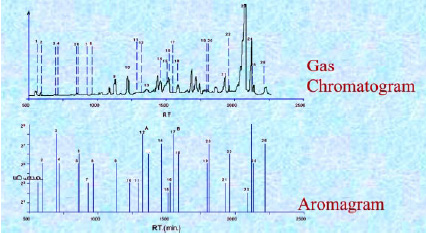
Producing the Aroma Compounds
The above techniques allow for the development of information needed to build an optimal flavor, but the flavorist will need the materials—the flavor ingredients—to do so. They can be either derived from nature or synthetically produced for application in artificial flavors.
Natural materials require significant effort. To isolate ingredients in a natural state requires some very sophisticated equipment. Three major areas of expertise exist to accomplish this task:
--- PAGE BREAK ---
• Supercritical Fluid Extraction. The first method utilizes equipment that extracts volatile organic materials by supercritical liquid carbon dioxide. Fig. 5 represents a typical flow diagram for the extraction of flavor ingredients by supercritical fluid extraction with carbon dioxide (Nakatasu et al., 2000). This is done at low temperature and high pressure (step 1) so that there is no heat damage to the natural aroma complex being extracted (step 2). There is no heat involved in the removal of the solvent (carbon dioxide) as it is released as a gas (step 3). The extract has a high-quality aroma profile, with many of the natural nuances. 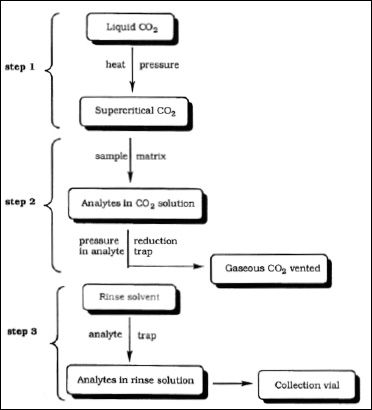
• Spinning Cone Extraction. A second method uses the commercially available Spinning Cone Column (Fig. 6; Flavortech Pty. Ltd., Griffith, NSW, Australia). In this method, the aroma components can be removed from a dilute water solution (essences) or slurry of the food, herb, or spice. The “spin cones” spread out the solution in a near monolayer, allowing for very efficient removal of the volatile flavor components. The distillation can be done in an inert atmosphere with a very low amount of heat because of the very efficient mass transfer allowed by the design of the equipment.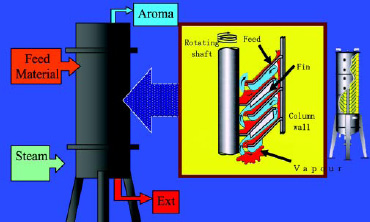
All types of food, herbs, and spices can be processed by the two methods discussed above, providing commercial yields of natural flavor materials.
• Enzymatic or Chemical Methods. Production of natural materials by enzymatic action or fermentation mimics nature, where natural enzymes or microorganisms are used to transform food materials into high levels of aromatic components. Various food materials, such as vegetable fats, and various enzymes and microorganisms can be used.
One important area for our company is the manufacture of dairy flavor ingredients. For example, ketones, particularly the C3 to C11 methyl ketones, are of great significance in adding the flavor character of blue or Camembert cheese to a flavor creation. They are also used at low levels to give the creamy notes typically found in milk and cream. These flavor compounds are formed from the hydrolysis of milk lipids using certain lipases. We use various combinations of enzyme treatments and extraction procedures to form and extract key flavor components.
l-Menthol is used in flavors because of its more desirable flavor profile and cooling effects. It is possible to produce l-menthol from l-menthone that appears in too-high levels in peppermint that is harvested too early. The conversion can be accomplished by the microorganism Pseudomonas putida (Trivedi, 1986).
Such methods are required if the final flavor is to be considered a “natural one.” FDA regulations (Code of Federal Regulations, Title 21, Section 101.22) indicate that the use of enzymes or microorganisms to ferment, convert, or express a flavor ingredient from a natural food, herb, or spice or part thereof is a natural process and the ingredient produced this way is considered natural.
Chiral Chemistry
Another method of producing these key characterizing aroma chemicals is through chiral chemistry.
--- PAGE BREAK ---
Nature produces chemicals in very specific shapes. Many common aroma chemicals can exist in one of several isomeric forms or as mixtures of them. When the molecular structures of two isomers are mirror images of one another, like our left and right hands, they are said to be enantiomers of each other or an enantiomeric pair of isomers. Molecules that cannot be super-imposed on their mirror image, such as enantiomers, are called chiral molecules. Chiral aroma chemicals—which polarize light in different ways and therefore have been referred to historically as optically active materials—have unique and interesting characteristics and properties.
An example of such a pair of isomers is d-carvone and l-carvone. Fig. 7 indicates the significant difference in odor character between the two enantiomers. These isomers have identical physical properties (molecular weight, boiling point, vapor pressure, etc.) but very different olfactive qualities—d-carvone has an odor reminiscent of caraway and dill, while l-carvone has an odor most closely associated with that of spearmint.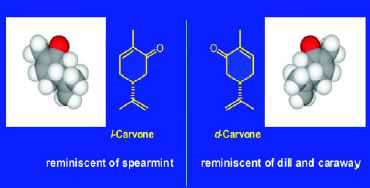
The differentiation of enantiomers requires a chiral discriminator, such as the nose for odors or taste buds for flavor materials. In the lab, the separation of enantiomers can be accomplished by chiral chromatography. There are a variety of analytical techniques, such as gas or liquid chromatography, which can perform this task. However, both techniques require a chiral stationary phase to afford discrimination or separation of materials.
Often in nature, materials such as carvone will be biosynthesized as a single enantiomer or as mixture of d and l forms, with one in excess. In contrast, chemical synthesis most often produces only an equal mixture of the two enantiomers. However, through highly advanced chemical synthesis technology known as catalytic asymmetric synthesis, or asymmetric catalysis, we have been able to imitate nature and produce materials which consist of only a single enantiomer. The resulting flavors that use these materials may be selected because of their nature-like profiles to be added to our Vivid Flavors™ line.
This technology selectively produces optically active chiral materials by using BINAP rhodium or ruthenium catalysts (Iwata, et al., 2002). These catalysts mimic the selectivity of enzymes in the chemical processes of nature. The commercial success of this technology is the result of a collaboration of more than 25 years between Takasago and Ryoji Noyori, a member of Takasago’s Board of Directors since 2000, who was jointly awarded the 2001 Nobel Prize in Chemistry for his pioneering work on asymmetric catalysis. Takasago and Professor Noyori have been granted more than 80 joint patents worldwide covering various aspects of this technology (Noyori et al., 1989, 1996; Ojima, 1993).
Our first commercial success using asymmetric catalysis was the industrial production of l-menthol, a material prized for its odor, flavor, and cooling sensory properties. It was industrialized in 1983 for the first time in the world through joint research by Nagoya University, Osaka University, and Takasago International Corp.
Menthol has three chiral carbon centers and hence eight possible stereoisomers or four pairs of enantiomers (Fig. 8). It is an excellent example of the important role stereochemistry plays in the odor, taste, and cooling properties of an aroma chemical. Of the eight possible stereoisomers, l-menthol is the predominant natural product and also the preferred material for taste, odor properties, and cooling effect. Fig. 9 shows the significant differences of thresholds for flavor, cooling, and bitterness that the various isomers of menthol possess. For example, the cooling effect and bitterness threshold of l-menthol are lower than those of d-menthol. Hence, less l-menthol is required to obtain the desired amount of cooling than d-menthol, and there is less bitterness. Cooling is one of the major attributes of menthol, and flavorists agree that the naturally occurring l-menthol is the superior isomer for use as a flavor ingredient. The industrial production of chiral l-menthol is accomplished by the asymmetric catalysis technology (Noboru and Matsumoto, 2002).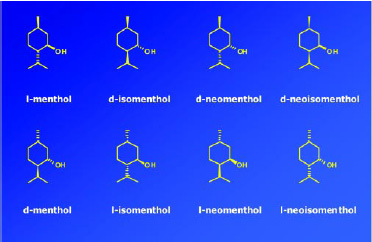
Chiral aroma chemicals offer significant environmental advantages, as well. In many instances, a single enantiomer is biodegraded much more efficiently than its mirror image. Therefore, the use of these chiral materials can significantly reduce the impact that flavors and their manufacture have on the environment by being more readily metabolized by microorganisms in the environment and in the human body.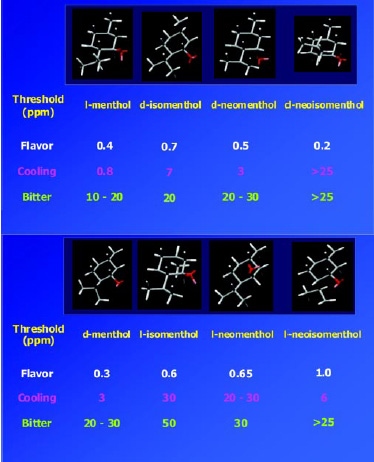
--- PAGE BREAK ---
Chiral aroma chemicals continue to usher in a new era of flavor and fragrance creation, providing materials with unique and interesting olfactory, sensory, and safety profiles simply not available in the past.
A wide range of chiral aroma chemicals for flavor and fragrance applications have been produced by asymmetric catalysis and used to produce flavors with unique sensory and flavor-enhancing properties. One such sensate material is 4-(l-men-thoxy-methyl)-2-(3´-methoxy-4´-hydroxyphenyl)-1,3-dioxolane, trade named HotAct® 1MM, which has been found to deliver an extreme sense of heat in the oral cavity (Nakatsu et al., 1996). Fig. 10 shows the molecular structure of one of its isomers superimposed on capsaicin, the pungent principle of chili peppers. This is another example illustrating the importance of chirality and its relation to flavor perception. The two materials interact similarly with the taste receptor responsible for hot or pungent effects. It is worth noting that sensate materials, materials that provide senasations such as cooling, warming, and tingle, are important aspects of flavors and typically don’t contribute directly to the aroma profile of the flavor.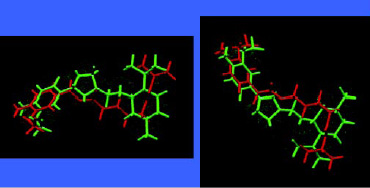
Verifying Superiority
Producing a final flavor by the total utilization of all these technologies requires a significant amount of work and perseverance in itself, but there is one last hurdle—the verification by sensory panels that the flavor is superior to those currently in the marketplace or is close to that of the original fruit or food product. The evaluation of the flavor by both expert panels and lay panels in a food or beverage application will facilitate the creation of a detailed profile of the flavor. At the same time, application experts will be evaluating the use of the created flavor in various formulations and measuring their shelf life.
Carter Green, Fred Pucarelli, Amby Mankoo, and Charles Manley
Author Green is Scientific Project Manager, Takasago’s Global Sensoral Center™; author Pucarelli is Director, Analytical Flavor Research, R&D Div.; author Mankoo is Vice President, Creative Flavor Research, Flavor Div.; and author Manley, a Past President and Professional Member of IFT, is Vice President, Science and Technology and Director of the Global Sensoral Center, all at Takasago International Corp (USA), 4 Volvo Dr., Rockleigh, NJ 07864. Send reprint requests to author Green at [email protected].
References
Abraham, M.A. and Sunol, A.A. 1997. “Supercritical Fluids. Extraction and Pollution Prevention.” Am. Chem. Soc., Washington, D. C.
Chinn, J.W. Jr. and Nakatsu, T. 1997. Odor collecting apparatus. U.S. patent 5,965,803.
Enberger, R. and Hopp, R. 1988. Synthesis and sensory characteristics of menthol enantiomers and their derivatives for the use of nature-identical peppermint oils. In “Topics in Flavor Research,” ed. R.G. Berger, S. Nitz, and P. Schrier, pp. 201-218, H. Eichorn, Marzling, Germany.
Iwata, T., Okeda, Y., and Hori, Y. 2002. Process for producing isopulegol. European patent 1,225,163.
Nakatsu, T., Lupo, A.T. Jr., Chinn, J.W. Jr. , and Kang, R.K. 2000. Biological activity of essential oils and their constituents. Chpt. 21 in “Studies in Natural Products Chemistry, Bioactive Natural Products (Part B),” ed. A. Rahman, pp. 571-580, Elsevier, New York.
Nakatsu, T., Green, C.B., Reitz, G.A., and Kang, R.K. 1996. 4(1-Menthoxymethyl)-2-phenyl-1,3-dioxolane or its derivatives and flavor composition containing the same. U.S. patent 5,545,424.
Noburu, S., and Matsumoto, T. 2002. Method for producing l-menthol. U.S. patent 6,342,644.
Noyori, R., Okuma, M., and Kumobayashi, H. 1989. Preparation of optically active 4-halo-3-hydroxybutyric aicd esters and carnitine. Japanese patent 01211551.
Noyori, R., Masahito, K., and Makoto, T. 1996. Preparation of optically active hydroxyalkylphosphonic acids as fosfomycin intermediates. Japanese patent 08012690.
Trivedi, N. 1986. Use of microorganisms in the production of unique ingredients. In “Biotechnology in Food Processing,” ed. S.K. Harlander and T.P. Labuza, Noyes Publishing, Park Ridge, N.J.
