Analyzing for Resistant Starch
LABORATORY
There is general consensus among public health authorities and nutritionists that the inclusion of fiber in the human diet provides health benefits. That benefit message has reached consumers, and many food and beverage companies have responded by launching products fortified with fiber.

According to E. Terry Finocchiaro ([email protected]), Director of Nutrition R&D at National Starch Food Innovation, Bridgewater, N.J. (www.nationalstarch.com), food product development professionals who have worked with fiber know that it can be difficult to formulate with fiber, but many may not appreciate just how difficult it may be to accurately analyze for it. Accurately measuring the fiber content of their foods and beverages is critical to making a sound benefit claim, whether it is a nutrient content claim, structure–function claim, or health claim.
One source of dietary fiber that is receiving increased interest for use as a food ingredient is resistant starch (RS), starch that resists digestion and absorption in the small intestine. Finocchiaro said that there are four types of resistant starch: “RS1,” physically inaccessible or indigestible starch, found in seeds, legumes, and unprocessed whole grains; “RS2,” starch that occurs in its natural granular form, such as uncooked potato, green banana flour, and high-amylose corn; “RS3,” starch with digestion-resistant crystalline regions formed when starch-containing foods are cooked and cooled, e.g., cooked-and-chilled potatoes or retrograded high-amylose corn; and “RS4,” chemically modified starches not found in nature, including starch ethers, esters, and cross-bonded starches.
National Starch, the leading supplier of natural resistant starches, offers a selection of resistant starch products that are derived from high-amylose corn starch, including Hi-maize® whole-grain corn flour (RS1 and RS2), Hi-maize 260 corn starch (RS2), and Novelose® 330 (RS3) resistant starch. The company also has a Web site devoted to resistant starch (www.resistantstarch.com).
Although the resistant starch types mentioned above generally refer to starch-based materials that are largely insoluble, there is a fifth type of soluble polysaccharide called “resistant maltodextrins,” Finocchiaro said. They are derived from starch that is processed to purposefully rearrange starch molecules to render them soluble and resistant to digestion. Two commercial resistant maltodextrins are Nutriose® from National Starch and Fibersol® 2 from Matsutani (www.matsutani.com).
Establishing Definitions and Seeking Methods
AOAC International (www.aoac.org) in the early 1980s reached consensus on a definition for dietary fiber—defined by Hugh Trowell and colleagues as “the plant polysaccharides and lignin which are resistant to hydrolysis by digestive enzymes of man”—and adopted Official Methods 985.29 and 991.43 for the determination of total dietary fiber. Since then, additional methods for dietary fiber components, including resistant starch, have been developed and validated by both AOAC International and AACC International (www.aaccnet.org). Today, there are numerous methods covering all types of soluble and insoluble fibers and resistant starches, maltodextrins, and dextrins.
--- PAGE BREAK ---
In 2001, the Institute of Medicine’s Panel on the Definition of Dietary Fiber responded to the Food and Drug Administration’s request to provide an updated definition of dietary fiber. In its report, Dietary Reference Intakes: Proposed Definition of Dietary Fiber (www.nap.edu/openbook.php?isbn=0309075645), the panel defined total fiber as the sum of dietary fiber and functional fiber. It defined dietary fiber as “nondigestible carbohydrates and lignin that are intrinsic and intact in plants,” where nondigestible means not digested or absorbed in the human small intestine, and functional fiber as “isolated, nondigestible carbohydrates that have beneficial physiological effects in humans.” The panel recognized that adoption of these definitions would require changes to the current analytical methods and proposed modifications to them.
In November 2007, FDA issued an Advanced Notice of Proposed Rulemaking, “Food Labeling: Revision of Reference Values and Mandatory Nutrients” (www.cfsan.fda.gov/~acrobat/fr071102.pdf), that cited the IOM report and set a deadline of January 31, 2008, for comments on analytical methods. In the meantime, for regulatory purposes regarding nutrition labeling, FDA considers dietary fiber as the material isolated using AOAC Method 985.29 or 991.43.
The Codex Alimentarius Commission’s Committee on Nutrition and Foods for Special Dietary Uses at its meeting in November 2008 adopted a new definition of dietary fiber for inclusion in the Guidelines on Nutrition and Health Claims. The committee defined dietary fiber as “carbohydrate polymers with 10 or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the small intestine of humans.” The committee also allowed local authorities to decide whether to include or exclude polymers with 3–9 monomeric units. The committee asked for information on methods to analyze fiber under the new definition to be submitted by March 1, 2009. The final report of the meeting (Alinorm 9/32/26) is available at www.codexalimentarius.net/web/archives.jsp.
According to Jonathan DeVries ([email protected]), Senior Technical Manager at Medallion Labs, Minneapolis, Minn., an interlaboratory study, coordinated by AOAC International and AACC International, is under way to adopt a validated dietary fiber method, applicable to all foods, that quantifies the fiber according to the Codex definition. The study is being conducted by a team of eight scientists in Ireland, France, Australia, Japan, and the United States, headed by Barry McCleary ([email protected]), CEO, Megazyme International Ireland Ltd., Wicklow, Ireland, and DeVries. The scientists, DeVries said, are combining the best aspects of AOAC-validated methods—991.43, 2001.03, and 2002.02—to produce a single method that provides an accurate measurement of the components in the Codex definition, including resistant starch.
When the validation phase of the study is completed, the results will be reviewed by technical committees in each organization for consideration as official/approved methods. In the meantime, the standard method for analysis of resistant starch is AOAC Official Method 2002.02/AACC Approved Method 32-40.
Analytical Methods
Finocchiaro anticipates increasing demand not only for fiber in general but also for resistant starch content. Many people want fiber in their diet, he said, and in many cases resistant starch provides more consumer-relevant health benefits than just “fiber” alone. According to Finocchiaro, the company’s customers in the food industry are recognizing that fact and wanting to promote resistant starch as a “super” fiber.
--- PAGE BREAK ---
National Starch customers run in-vitro assays, he said, because they need to validate the resistant starch or total dietary fiber content of their products for labeling purposes. If they make a simple nutrient claim, they must analytically validate the amount of total dietary fiber claimed on their labels. The company also invests heavily in clinical studies that will allow its customers to refer to the various structure/function claims enabled by such studies, he said, provided that the material in the product delivers the same dosage of resistant starch or total dietary fiber validated by the clinical studies. Consequently, there is increasing interest in resistant starch methodologies.
When looking at the analytical methods available, he said, it is important to understand their strengths and weaknesses. Some methods correlate well with theoretical values based on resistant starch content when analyzing ingredients, and others correlate well when analyzing food products fortified with resistant starch ingredients.
In developing methods, in-vitro results must be validated against in-vivo results. One of the ways to assay resistant starch in vivo, Finocchiaro said, is to determine the amount of undigested starch in the human ileum, the terminal portion of the small intestine, obtained by intubation or from ileostomy bags after consumption of a starch sample. The intubation technique overestimates resistant starch content, while the ileostomy model tends to underestimate it.
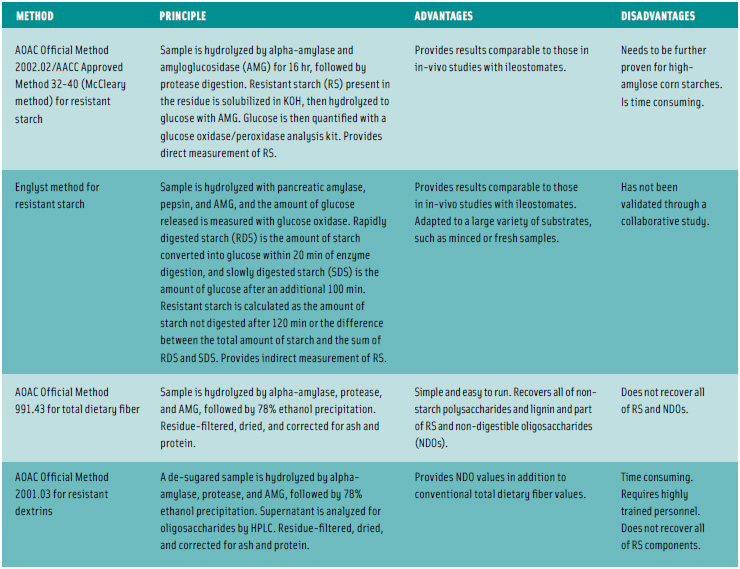
Most foods containing resistant starch in the U.S. are analyzed as total dietary fiber by AOAC Method 991.43, Finocchiaro said, but the issue of quantifying these materials as resistant starch becomes a bit more complicated and confusing. There are basically four in-vitro methods that are relevant in quantifying resistant starch in foods, and the selection of methodology requires careful consideration. Historically, he said, there have been only two methods for analyzing the classic, mainly insoluble, RS 1– 4 materials. The Englyst method, developed by Hans Englyst, has wide acceptance but has not been AOAC approved. The McCleary method, on the other hand, is approved as AOAC Official Method 2002.02; it also has wide acceptance in the fiber community.
A third, relatively new method, has been developed by Anthony Bird ([email protected]), Principal Research Scientist at CSIRO Human Nutrition, Adelaide, Australia, and undergone a pilot interlaboratory study establishing the operational performance of the method. Bird said that several ileostomy studies have provided resistant starch reference values for validating the laboratory assay. The method has been automated and patented, but details will not be available until the patent is published later this year. A fourth method, AOAC Method 2001.03, is worth noting, Finocchiaro said, as it quantifies soluble resistant maltodextrins; however, it is more complicated to run.
--- PAGE BREAK ---
The accompanying Table 1, prepared by Finocchiaro and Susan Cho, President of NutraSource, Clarksville, Md. ([email protected]), details the primary in-vitro methods available for analysis of resistant starch and total dietary fiber, along with their advantages and disadvantages. They all employ enzymatic digestion steps, Finocchiaro said, and can be complicated to run. They generally take a full working day to complete, and they have to be run at least in duplicate and carefully timed—you can’t stop the reaction for a lunch break. Consequently, he said, National Starch invests heavily in helping to validate its customers’ lab methods.
Conferences to Address Dietary Fiber
Several conferences to be held in the next few months will feature papers and symposia on dietary fiber:
• The 2009 IFT Annual Meeting & Food Expo, to be held in Anaheim, Calif., on June 6–9, will feature many papers and symposia on all aspects of food, including symposia on “Resistant Starch and Health” and “Carbohydrates and Health.” Details are available at www.am-fe.ift.org.
• The 2009 International Dietary Fibre Conference organized by the International Association for Cereal Science & Technology (ICC), to be held in Vienna, Austria on July 1–3, will feature papers and sessions on all aspects of dietary fiber, including a keynote address on resistant starch. Details are available at www.icc.or.at/events/df09.
• The 2009 AACC International Annual Meeting, to be held in Baltimore, Md., September 13–16, will feature many papers and symposia on all aspects of cereal chemistry, including symposia on “The Effects of Dietary Fiber from Cereals on Gut Health” and “What Scientific Evidence Is Needed to Substantiate Health Effects of Dietary Fiber From Cereals?” Details are available at http://meeting.aaccnet.org.
Neil H. Mermelstein, a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]
