Flavor Encapsulation: A Convergence of Science and Art
A variety of encapsulation processes are available to deliver and control the release of flavors in food systems. Here’s how they work.
Flavor encapsulation is the general term for the various processes employed to deliver liquid flavors in a standardized, functional form. The benefits of encapsulating flavors are numerous: converting a liquid flavor into an easily dispensable powder, protecting a specific flavor or key flavor components from change, delivering more flavor impact in a finished product, supplying visually distinct, flavored particles, and in some instances, providing controlled-release functionality in a product application.
Selection, development, and implementation of a specific encapsulation strategy is mostly hidden from the ultimate user. That technical strategy used by the encapsulator is in large degree based on the encapsulator’s prior experience, technical depth, proprietary knowledge, availability and scalability of process systems, and an ability to match an optimum system with a flavor.
There are only a few good literature articles (Risch and Reineccius, 1988; Ho et al., 1995; Gibbs et al., 1999) and no “technical bibles” readily available to detail the key strengths (and weaknesses) of each encapsulation system in any great depth. The patent literature remains the single best source of information regarding technical disclosures of specific encapsulation technologies, formulas, and processes.
Manufacturing processes used in flavor encapsulation, which often become by default the name descriptor, include spray drying, melt extrusion, melt injection (the “Durarome” process), spray chilling (lipid encapsulation), lipid coating, liposome formation, complex coacervation, absorption (plating), adsorption, complexation, and co-crystallization. While a large number of approaches and processes have been evaluated, only a few are ultimately utilized in commercial systems. Table 1 lists a number of these flavor-encapsulation technologies, their relative commercial use, and the key physical state for each.
Key Parameters
When reviewing the key parameters of any flavor encapsulation system, the role of the flavor and material state must be considered, not independently, but as an interactive, integrated system, in conjunction with the process.
• Flavor. The liquid flavor, the starting point for the encapsulation process, is a critical variable, but it is often overlooked in terms of the process. Flavors consist of tens to hundreds of aromatic, volatile organic compounds, some detectable at the parts-per-billion range. Very slight changes in a flavor composition profile can be detected by that most sensitive of analytical equipment, the human olfactory system. The flavor source can be a compounded flavor (either natural or artificial), a flavor essence, a flavor oil, an essential oil, a flavor extract, a reaction flavor, or combinations of these various flavor types. The active flavor principals are normally standardized to a specific strength with a co-solvent.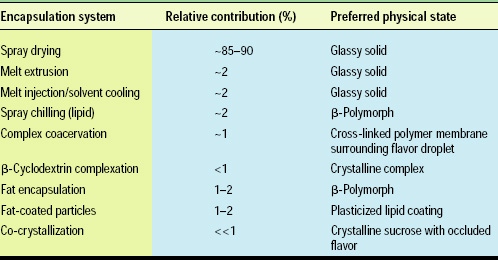
The choice of co-solvent will also greatly affect subsequent operations. Propylene glycol and ethanol are hydrophilic solvents used with fruit flavors, which are composed of low-molecular-weight esters, aldehydes, and alcohols, while fractionated coconut oil or medium-chain triglycerides are used with more hydrophobic flavors. The encapsulation specialist recognizes that some flavor chemicals—e.g., diacetyl (butter note), ethyl butyrate (fruity note), and butyric acid (cheese note)—will partition into the water and lipid phases if emulsions are intermediates in the encapsulation process.
Any process for generating encapsulated flavors must meet specific requirements. The first, obviously, is to produce material in high yields while retaining the original flavor profile. Moreover, process economics must not greatly increase the final cost of the product over comparable competitor products. Physical properties of the encapsulated material are optimized in terms of particle size, free-flowing and non-caking character, and formation of the glassy state (where applicable). Commercialization issues are a consideration only when a newly developed technology is first employed. Other items of consequence include regulatory constraints, availability of ingredients, use of newer unique ingredients, ingredient functional uniformity, and use of solvents or chemical agents.
--- PAGE BREAK ---
• Glassy State. The first three encapsulation systems listed in Table 1 constitute 90–95% of commercial encapsulated flavors; these encapsulated flavors are found in the preferred glassy state. The role of the glassy state in food systems has been a subject of increasing importance to the food industry. The pioneering work of Levine and Slade (1988, 1991) and Levine (2002) has shown the glassy state to be a key underlying physical-chemical basis for such diverse properties as powder stability, flavor encapsulation, and food product texture.
Elliott (1994) defines the glassy state as follows: “A glassy (or synonymously, vitreous) material is an amorphous solid that exhibits a glass transition. (Thus, by this definition, all glasses are amorphous, but not all amorphous solids are necessarily glassy.) The glass transition is marked (as a function of temperature) either by a change in slope of extensive thermodynamic properties (e.g. volume or entropy) or equivalently as a discontinuity in derivative quantities (e.g. specific heat or thermal expansivity).”
To generate carbohydrate-based glassy matrices, the original material must first be placed into an intermediate isotropic state (by either dissolution in water or melting) and rapidly cooled/dried to yield a low-moisture solid. The glassy state is then both a reflection of the process and the final composition.
The glassy state and the glass transition temperature are most easily determined by thermal analytical methods. Differential heat capacity ΔCp can be easily obtained by differential scanning calorimetry (DSC) or modulating differential scanning calorimetry (MDSC) to yield the ΔCp in units of Joules/(g-°K). The glass transition temperature Tg is by general convention the temperature at the midpoint of the transition of an amorphous solid going from the glassy amorphous state to the rubbery amorphous state.
Water-soluble flavor components can be solubilized within the carbohydrate glass matrix. Complex lipophilic flavor molecules are encapsulated as droplets dispersed within the glass matrix. These droplets can continue to show the reactivity of the original lipid flavor (interesterification, isomerization, oxidation, etc.) but are protected from additional oxidation by the diffusion barrier properties of the glass matrix.
Encapsulation Systems
There are six different primary types of encapsulation systems:
• Spray Drying. This process (Fig. 1) remains the dominant process technology of the microencapsulation field. The basics of the engineering process and various dryer configurations can be found in Masters (1979). The process starts with formation of an aqueous carrier phase. The flavor, in most cases an oil-soluble flavor in a lipid co-solvent, is emulsified in the aqueous carrier phase and fed into the hot-air plenum of the dryer chamber by an atomizing head and the particles immediately heated. A film forms at the droplet surface retarding the flavor molecule diffusion while allowing water molecules to rapidly diffuse and migrate to the surface and vaporize. By controlling the air-inlet temperature, emulsion feed rate, and evaporative cooling, the droplet temperature should never exceed 100°C. The dried particles are removed in a manner to prevent their overheating and “scorching.” Generally the spray-dried encapsulated flavor particles are obtained with a particle size of 10–150 microns.
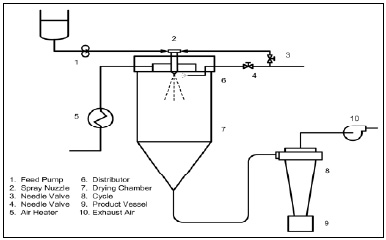
The requirements for an ideal spray-drying carrier include a high degree of solubility, limited viscosity at the 35–45% solution solids range, emulsifying properties, good drying properties, nonhydroscopic character, bland taste, nonreactivity, and low cost. Modified starches, also referred to as OSAN starches (n-octenylsuccinic anhydride–substituted starches), are used extensively.
--- PAGE BREAK ---
The first generation of these starches were prepared by spraying on the anhydride, then esterifying and dextrinizing the starch granule in a heated chamber. The result was a tan, modified, dextrinized starch with excellent emulsifying capacity and good solubility-viscosity properties in solution. One undesirable property was the presence of a detectable “cardboardy” off-note.
A second generation of OSAN starches are now replacing the original OSAN starches. These new starches utilize acid or enzymes to break down the starch to soluble oligomers which are then derivatized. These new products are white and very bland, and they retain the equivalent good emulsifying and drying properties.
The OSAN starches are relatively inexpensive, but they all have one major drawback: the associated n-octenylsuccinic acid group is very acidic and generates a pH of about 3.0 in the aqueous emulsion. Depending on the starch manufacturer’s processes or by subsequent autohydrolysis of the succinyl ester group, free n-octenylsuccinate levels greater than 0.1% can be encountered in the emulsion. This acidity and the free acid lead to the possibility of some flavor molecules undergoing unwanted acid-catalyzed changes such as cyclizations, hydrolyses, isomerizations, and esterification reactions.
Another standard carrier for spray drying is gum arabic. This hydrocolloid gum exhibits excellent emulsifying properties, high solubility, and low solution viscosity, and the dried powder is resistant to caking. Gum arabic’s film-forming property greatly assists the drying of many hygroscopic materials. The supply of this exudate gum had earlier been dependent on hand collection by nomads. The regional politics in central Africa and recent embargos greatly affected price and availability. Now commercial acacia tree “farms” have become the dominant source, stabilizing both supply and price.
Maltodextrins are bland, inexpensive, highly soluble, low-viscosity carriers. These oligomers, prepared by hydrolyzing starch, have no emulsifying properties and are more likely to be used with OSAN starch or gum arabic as a cost-reducing diluent.
Other food polymers described or patented as spray-drying carriers include milk proteins, larch gum (arabinogalactan), hydrolyzed gelatin, and modified celluloses, each with limited utility.
All the food polymer carriers can be dried into the glassy state. However, to improve glassy-state properties of the spray-dried product, the addition of lower-molecular-weight carbohydrates, e.g., sugars, corn syrup solids, and polyols, at 10–35% of the total carrier formula is recommended. This combination affects molecular packing, thereby increasing particle density and enhancing the “degree” of the glassy state.
Spray-dried encapsulated flavors are successfully employed in many food blends such as seasonings, and consumer products such as cake, cookie, and pudding mixes. However, in certain product systems, a spray-dried encapsulated flavor can lead to failure. For example, a spray-dried flavor may be added to a tea mix before the blend is placed in the tea bag. In this environment, the micron-sized flavor particles can ultimately shift through the paper barrier over time and be lost from the tea bag before use.
• Melt Extrusion. The melt-extrusion process (Fig. 2) utilizes a co-rotating twin-screw extruder to melt carbohydrate carriers, which are ultimately expelled under pressure through a die to form sheets, ropes, or threads of differing dimensions. The use of specific screw configurations and other proprietary modifications to the system are required to optimize running conditions and product properties (Zasypkin and Porzio, 2004). Rapid cooling of the extruded mass to a glassy state allows the process to be continuous as the glassy matrix is conveyed, milled, sifted, and packed off. Addition of small amounts of water as a plasticizer to the feed zone of the extruder must be balanced against its Tg-lowering effect to ensure that the final Tg of the product is >30°C. Encapsulant flavors can be added either at the feed port of the extruder or to the molten mass in the final zone using specialized pump systems. Some melt-extrusion encapsulated flavor systems are McCormick & Co.’s FlavorCellTM (Popplewell et al., 1995) and IFF’s Caplock®. Most of the recent melt-extrusion carrier-composition patents are held by McCormick & Co. (Black et al., 1998; Fulger and Popplewell, 1997, 1998, 1999; Popplewell et al., 2002; Porzio and Popplewell, 1997, 1999, 2000, 2001, 2002, 2003).
--- PAGE BREAK ---
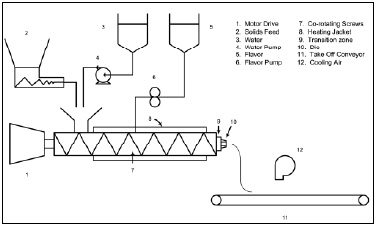
The particular advantage of the melt-extrusion glass encapsulated products is the ability to supply larger flavor particles for visual impact in products. For example, colored and flavored particles have been used in hard candies and personal-care tablets (Popplewell et al., 1995). In addition, the larger flavor particles can dissolve slowly and have exhibited some protection and controlled-release properties in specific bake mixes, batters, and fried-snack half-products. Use of specific, higher-molecular-weight food polymers in the larger particles has extended protection (and exhibited some controlled release) in selected product applications.
• Melt Injection. This process, often referred to as the “Durarome” process after the product trade name, consists of making up a sugar syrup (or a sugar–corn syrup-solids syrup) and boiling off significant amounts of the remaining water. Flavor oils are then added to the hot molten sugar, the pressure vessel closed, and high-shear mixing employed to emulsify the flavor oil. The vessel is pressurized, and the hot sugar emulsion is expelled through fine orifices into a chilled solvent bath. The solvent, almost exclusively isopropanol, simultaneously chills and dehydrates the sugar strands. After filtering the glassy rods from the chilling bath, the free solvent must be removed and residual solvent driven off. The product is obtained as fine threads free of surface oil. This property is especially important when citrus oils are to be encapsulated. Oxidation of surface oil limonine (the major terpene in citrus oil) to limonine epoxide is very rapid and can contribute to a distinct unacceptable flavor off-note.
Although all the patents teaching the process and carrier compositions for melt injection have expired and are in the public domain, only one or two flavor houses now utilize this process. This is due in part to the large startup capital cost, environmental and safety issues in handling solvent, the hydroscopicity of the sugar matrix, and the inefficiency of batch processing. However, the early success of these encapsulated flavors in instant drink mixes established the product as a standard in the industry, and it still remains an item of commerce.
• Complex Coacervation. This encapsulation system is based on the controlled phasing out from solution of two interactive water-soluble polymers to form a mixed-polymer coacervate film around a lipid droplet. First developed in the 1950s by National Cash Register Co., it was the basis for carbonless copy paper. Application to the food industry has been limited since many water-soluble flavors and interfacially active flavor components can inhibit the coacervate wetting and coating dynamics at the lipid–water interface.
Careful control and adjustment of pH, temperature, and polymer concentrations are required for a successful coacervation process. Food regulations limit the use of glutaraldehyde as a cross-linking agent to the gum arabic–gelatin coacervate pair (FDA, 2003). A newer nonchemical approach to crosslinking the protein component employing transaminase enzymes may open up new options for use in the food processing industry. At present, only one major flavor house produces complex coacervated encapsulated flavor products.
• Lipid Encapsulation. Presently, there are three forms of commercial lipid-encapsulated materials. In the first, a molten lipid–flavor system is prepared, atomized as fine droplets into a chilled atmosphere chamber, solidified, and recovered as fine particles. This lipid-encapsulation process is referred to as “spray chilling,” where the term lipid is understood. An alternate approach is to dissolve the flavor in the molten lipid and solidify it on a chilled drum surface. The resulting solid is obtained as larger flakes. Both processes depend on the selection of the proper fats and oils to yield a solid at room temperature in the preferred triglyceride polymorphic form and with the desired melt-release properties.
Another process entails protecting food solids with a lipid coating. Particles such as crystalline acids, salts, minerals, or vitamins are coated to ensure protection from moisture or for thermal release at specific temperatures. These solid particles are coated with a minimum level of the molten lipid necessary to ensure a mechanically stable, continuous covering. The coating process is most likely accomplished in a fluidized-bed system.
--- PAGE BREAK ---
• β-Cyclodextrin Complexation. The formation of a 1:1 crystalline molecular complex (Fig. 3) of specific flavor molecules with β-cyclodextrin (a 7-member cyclic glucosal oligomer also known as a Schardinger dextrin) is based on size–shape molecular interactions. The β-cyclodextrin molecule can be envisioned as a donut-shaped molecule with a hydrophobic interior cavity. The ring has the proper dimensions to form a complex with specific organic flavor molecules. With a linear geometry and proper molecular dimensions, the flavor can fit into the cavity, forming a molecular complex and thereby changing the overall solubility of the agents and promoting crystallization from solution. This complex has been described as a “finger in the donut.”
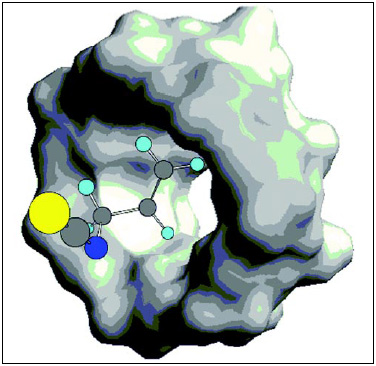
The critical molecular geometry requirements can lead to selective complexation from the complex mixture that constitutes a flavor oil or extract. The molecular weights of the cyclodextrin and flavor molecule set an upper limit of the flavor component at 10–11% by weight in the flavor complex. Preparation of the complex entails warming an aqueous solution to increase solubility of the β-cyclodextrin, followed by addition and mixing of the flavor, holding and cooling to form the complex, then filtering and drying.
Allylisothiocyanate, the heat principle found in mustard oil, wasabi, and fresh horseradish, is an ideal flavor molecule for β-cyclodextrin encapsulation. It is a low-molecular-weight, linear molecule exhibiting significant volatility and chemical reactivity in the liquid state at room temperature. The molecule can undergo significant changes, including isomerization, oxidation, and polymerization in the neat (pure liquid) state. (It is also a potent lachrymator and can be classified as a chemical warfare agent). Formation of the allylisothiocyanate–β-cyclodextrin complex yields a stable, crystalline powder that only releases the isothiocyanate in the presence of moisture. Recently, the γ-cyclodextrin has been self-certified as generally recognized as safe (GRAS), and the α-cyclodextrin is scheduled to follow in the near future (Reusher, 2003).
Controlled Release and Flavor Delivery
The term controlled release with regard to encapsulated flavors has as many interpretations as there are applications. In a simple case, a spray-dried flavor used in a seasoning exhibits controlled release when the seasoning (coated on the snack) is eaten. In a more-complex food system, controlled release can mean release during baking but not during the initial preparation of a cake mix by the consumer. In a much more sophisticated product use, controlled release may mean the repeated release of extremely volatile flavors from an instant coffee product on repeated openings of the container (Zeller et al., 1987).
At the extreme is the occasional request that a flavor house may receive from a cereal manufacturer for an encapsulated flavor that can be added to the cereal base, survive the cereal processing, and then be released by milk in the bowl. If the product is a puffed cereal, the encapsulated flavor would have to survive the steps of cereal cooking/extrusion (moisture, mechanical, and thermal stressors), pelletizing (heat, drying), and gunning (severe heating, flashing steam). Obviously, that degree of controlled release is beyond the flavor industry’s current capabilities. It would be more practical to have the manufacturer add the flavor at the end of the total process. Delivery and controlled release of an encapsulated flavor are always determined by the environments involved with the preparation and final state of the food product.
Future Opportunities
The demand for new flavor-delivery vehicles, improved-tasting products, and more-cost-effective technologies will continue to challenge the large flavor houses and specialty encapsulation companies. The extensive number of sophisticated controlled-release technologies developed by the pharmaceutical industry will not likely transfer easily to the food industry. Key differences in regulatory and functional requirements of the two industries as well as the cost–benefit economics will keep technology transfer at a minimum.
The food industry must draw on the increasing sophistication found in the fields of materials science, polymer science, physical chemistry (phase diagrams, adsorption dynamics, interfacial and colloid chemistry, glassy state), sensory, flavor chemistry (isolation, characterization, synthesis, compounding, and reaction flavors) and process engineering as a starting point for new encapsulation opportunities. These opportunities might include such new approaches as hyphenated technologies, multiple-path systems, nanotechnology, and the melding of flavor-enhancement technologies with flavor-encapsulation systems.
--- PAGE BREAK ---
• Hyphenated Technologies are the combination of two encapsulation technologies to augment the benefits of each. These hyphenated systems might include spray drying–melt extrusion, spray drying–liposomes, complex coacervation–spray drying, fat coating–extrusion, spray chilling–water-in-oil microemulsions, melt extrusion–β-cyclodextrin, complexes, etc.
One key to the use of hyphenated technologies will be the issue of benefits vs costs required by the double processing. Activity-based costing (ABC), the new religion of financial control agents, is inclined to work against these newer encapsulation systems.
An interesting example of a mixed encapsulation technology is found with a coacervate–spray drying system. Fig. 4 shows the intermediate in that process—a multiphase emulsion consisting of a flavor oil, a polymer-rich coacervate phase, and a second immiscible aqueous polymer-poor phase. The total dispersed system had additional carrier solutes added and was then spray dried.
• Multiple-Path Processes are alternate options to optimizing spray-dried flavors by individually processing selected components. Under normal circumstances, a flavor is supplied in complete form for encapsulation. In specific cases of compounded flavors, the highly volatile components may be separated and spray dried with selectively matched carriers. For example, fresh allium, vegetable, and fruit flavors contain significant amounts of the added volatile top note, acetaldehyde. Spray drying this isolated acetaldehyde is better suited to a non-emulsifiying, neutral, high-soluble-solids aqueous carrier which yields an optimized glassy matrix in the dried form. A number of systems have been directed at this objective (Porzio, 2003). Process safety issues do arise with this volatile and potentially explosive chemical vapor. With successful encapsulation into the glassy state, the solid form of acetaldehyde can be added back to the original, modified encapsulated flavor to match the equivalent percentages in the final flavor formula.
• Nanoparticle Technology is the general term for the unique properties of submicron-sized particles. In flavor delivery, these particles can be generated by liquifying a high-melting fat, forming the appropriate submicron-sized lipid droplets in an emulsion, and cooling the lipid phase to solidify lipid nanoparticles.
The use of nanoparticles in flavor encapsulation has been described recently by Shefer and Shefer (2003). However, nanotechnology may have a another future in the form of “nanopored” particles as a novel adsorbent encapsulation matrix. Based on the Kelvin equation, capillary condensation of a volatile occurs as the flavor vapor phase forms a reduced-partial-pressure condensed state in capillary pores. By preparing highly porous carbohydrate polymers with appropriate pore diameters, it may be ultimately possible to completely reduce the partial vapor pressure of key flavor, extremely volatile, top notes such as dimethyl sulfide (cooked corn note) or acetaldehyde (fruit top note) and render them stable by capillary condensation at room temperature. An interesting execution toward this approach is given by Buttery et al. (1999).
• Flavor-Enhancing Technologies which can be married to standard encapsulation technologies will be another “outside-the-box” approach to encapsulation and flavor delivery. The cellular neural receptors and molecular signal-transduction pathways involved with flavor perception may now be activated and amplified using newly discovered and known flavor-enhancing agents. As a result, flavors will be perceived physiologically as more intense.
Today, the orthodoxy regarding physiological perception of aromatic scent (and flavor volatiles) is being challenged. Turin (1996) proposed a new theory, that olfactory receptors respond not to the shape of the flavor molecules but to their vibrations. The phenomenon of inelastic electron tunneling is the proposed mechanism of the biological transduction. Turin’s theory explains a number of unrelated issues involved in olfaction, including isotope effects, the role of zinc in the olfactory receptors and in anosmia, the puzzle of the carvone enantiomers, the prediction of B–H (borane) bonds having identical odor character as S–H (sulfhydryl) groups, and the correct prediction that ferrocene (dicyclopentadienyl iron) and nickelocene (dicyclopentadienyl nickel), which have identical shape and size and encase dissimilar metal ions, have distinctly different odor character. His ultimate conclusion is that olfaction, like color vision and hearing, is a spectral sense. Burr (2002) tells of Turin’s journey in researching, developing, testing, then attempting to publish his theory and the resistance of the fragrance industry to that theory.
The flavor-encapsulation field will continue to require a more-sophisticated understanding of flavor–material interactions in the pursuit of new delivery technologies. In the end, it is the physical-chemical properties of the aromatic flavor components which dominate the technology selection, the encapsulation processes, and the degrees of freedom available to the encapsulator in crafting new flavor-delivery systems.
The author, a Professional Member of IFT, is Distinguished Scientist, Technical Innovation Center, McCormick & Co., Inc., 202 Wight Ave., Hunt Valley, MD 21031 ([email protected]).
References
Black, M., Popplewell, L.M., Porzio, M.A. 1998. Controlled-release encapsulation compositions. U.S. patent 5,756,136.
Burr, C. 2002. “The Emperor of Scent.” Random House, New York.
Buttery, R.G., Glenn, G.M., and Stern, D.J. 1999. Sorption of volatile flavor components by microcellular cereal starch. J. Agric. Food Chem. 47: 5206-5208.
Elliott, S.R. 1996. Defects and disorder in crystalline and amorphous solids. In “Amorphous Solids: An Introduction,” ed. C.R.A. Catlow, pp 73-85. Kluwer Academic Publishers, the Netherlands.
FDA. 2003. Microcapsules for flavoring substances. Food and Drug Admin., Code of Federal Regulations, 21 CFR 172.230.
Fulger, C.V. and Popplewell, L.M. 1997. Flavor encapsulation, U.S. patent 5,601,865.
Fulger, C.V. and Popplewell, L.M. 1998. Flavor encapsulation, U.S. patent 5,792,505.
Fulger, C.V. and Popplewell, L.M. 1999. Flavor encapsulation, U.S. patent 5,958,502.
Gibbs, B.F., Kermasha, F., Alli, I., and Mulligan, C.N. 1999. Encapsulation in the food industry: A review. Intl. J. Food Sci. Nutr. 50: 213-224.
Ho, C.-T., Tan, C.-T., and Tong, C.H. 1995. “Flavor Technology—Physical Chemistry, Modification and Process.” ACS Symp. Series 610. Am. Chem Soc., Washington, D.C.
Levine, H. 2002. “Amorphous Food and Pharmaceutical Systems.” Royal Society of Chemistry, Cambridge, U.K.
Levine, H. and Slade, L. 1988. Thermomechanical properties of small carbohydrate-waterglasses and rubbers. J. Chem. Soc. Faraday Trans. 184: 2169-2633.
Levine, H. and Slade, L. 1991. “Water Relationships in Foods.” Plenum Press, New York.
Masters, K. 1979. “Spray Drying Handbook.” John Wiley and Sons Inc., New York.
Popplewell, L.M., Black, J. M., Norris L., and Porzio, M. 1995. Encapsulation system for flavors and colors. Food Technol. 49(5): 76-78, 80, 82.
Popplewell, L.M., Black, M.J., Madsen, M. G. 2002. Cake-resistant, hygroscopically sensitive materials and process for producing the same. U.S. patent 6,444,246.
Porzio, M.A. 2003. Flavor delivery, microencapsulation and the glassy state. Presented at Ann. Mtg., Inst. of Food Technologists, Chicago, Ill., July 12-16.
Porzio, M.A. and Popplewell, M.L.1997. Encapsulation compositions. U.S. patent 5,603,971.
Porzio, M.A. and Popplewell, M.L.1999. Encapsulation compositions. U.S. patent 5,897,897.
Porzio, M.A. and Popplewell, M.L. 2000. Salt compositions and method of preparation. U.S. patent 6,090,418.
Porzio, M.A. and Popplewell, M.L. 2001. Encapsulation compositions. U.S. patent 6,187,351.
Porzio, M.A. and Popplewell, M.L. 2002. Encapsulation compositions. U.S. patent 6,416,799.
Porzio, M.A. and Popplewell, M.L. 2003. Encapsulation compositions. U.S. patent 6,652,895.
Reusher, H. 2003. Cyclodextrins: Microencapsulation at the molecular level. Presented at Ann. Mtg., Inst. of Food Technologists, Chicago, Ill., July 12-16.
Risch, S. and Reineccius, G. 1988. “Flavor Encapsulation.” ACS Symp. Series 370. Am. Chem. Soc., Washington. D.C.
Shefer, A. and Shefer, S. 2003. Novel encapsulation system provides controlled release of food ingredients. Food Technol. 57(11): 40-42.
Turin, L. 1996. A spectroscopic mechanism for primary olfactory reception. Chem. Senses 21: 773-791.
Zasypkin, D. and Porzio, M. 2004. Glass encapsulation of flavors with chemically modified starch blends. J. Microencapsulation (in press).
Zeller, B.L., McKay, R.P., and Saleeb, F.Z. 1987. Method and manufacture for moisture-stable, inorganic, microporous saccharide salts. U.S. patent 4,659,390.
ADDITIONAL READING
Balassa, L.L. and Fanger, G.O. 1971. Microencapsulation in the food industry. CRC Crit. Rev. in Food Technol. l: 245-265. Dated, but notes advantages/disadvantages with various systems.
Gibbs, B., Kemasha, S.,Alli, I., and Mulligan, C.N. 1999. Encapsulation in the food industry: A review. Intl. J. Food Sci. Nutr. 50: 213-224. Very brief review.
Jackson. L. and Lee, K. 1991. Microencapsulation and the food industry. Lebensm. Wiss. u. Technol. 24: 289-297. Good review on carriers, process, encapsulants.
Shahidi, F. and Han, X.-C. 1993 .Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 33: 501-547. Good, extensive review.
Shun, P.L., Kowarski, C.R., Feld, K.M., and Grim, W.M. 1988 Recent advances in microcapsulation technology and equipment . Drug Dev. Ind. Pharm. 14: 353-376. Pharmaceutical process and equipment oriented.
Sparks, R.E. 1981 Microencapsulation. In “Kirk-Othmer: Encyclopedia of Chemical Technology,” 3rd ed., Vol. 15, pp. 471-493. Dated but well written.
Thies, C. 1987. Microencapsulation. In “Encyclopedia of Polymer Science and Engineering,” ed. J. Kroschwitz, 2nd ed., Vol. 9, pp. 724-745, John Wiley & Sons, Inc., New York. Excellent.
Thies, C. 1994. Microencapsulation: Minor answer to major problems. Today’s Chemist, Nov., pp. 40-45. Short and simplified.
Uhlemann, J., Schleifenbaum, B., and Bertram, H-Z. 2002 Flavor encapsulation technologies: An overview including recent developments. Perfumer Flavorist 27(5): 52-61. Focuses on equipment and uses many visual schematics.
Zeller, B.L., Saleeb, F.Z., and Ludescher, R.D. 1999. Trends in development of porous carbohydrate ingredients for use in flavor encapsulation. Trends Food Sci. Technol. 9: 389-394. Specialized re glassy state in matrix.
