Improving the Sanitation of Food Processing Surfaces
Nanoscale modification of surfaces can produce antimicrobial properties that can be recharged through sanitizing.
As food production becomes increasingly automated, the number of surfaces with which foods come into contact and the subsequent potential for contamination increase (Reij and Den Aantrekker, 2004; Todd et al., 2010). Cross-contamination of foods can occur when microorganisms that contaminate the surface of a material like stainless steel or plastic are transferred to food.
Such contamination can occur during harvest, processing, and post-processing and may result in outbreaks of foodborne illness and loss of product due to spoilage or recalls. Even fresh and minimally processed produce comes in contact with a number of materials in the farm-to-fork continuum from picking buckets to rinsing bins, packing tables, handling, and display cases.
Indeed, product recalls and outbreaks of foodborne illness highlight the motivation to reduce incidences of contamination. In addition to the public health concern posed by the transfer of foodborne pathogens, contamination by food spoilage organisms causes a significant economic impact and results in lost food quality. Food processors are therefore well aware of the importance of properly cleaning and sanitizing food contact surfaces such as valves, gaskets, work tables, and conveyor belts in order to maintain safe and high quality product.
What is becoming increasingly clear, however, is that it is not just food contact surfaces that can pose a risk of contamination. Survival of pathogenic and spoilage organisms on non-food contact surfaces is a continuing challenge to food safety and food quality. Contamination by microorganisms that survive on personal protective equipment, in drains, on hoses, in air-handling systems, or on doorknobs are also a potential source for transfer onto finished products.
The economic burden due to foodborne illness was recently quantified as $152 billion annually (Scharff, 2010). The financial impact of product loss due to food spoilage organisms is likely quite high as well. Therefore, a continuing need exists to improve the safety and sanitation of food processing materials, including both food contact and non-food contact surfaces. Antimicrobial materials have the potential to increase food safety. An emerging class of antimicrobial materials called N-halamines are particularly of interest because of their ability to regenerate antimicrobial properties following use of certain sanitizing agents.
--- PAGE BREAK ---
Antimicrobial Materials Development
The number of commercially available antimicrobial materials for contact surfaces continues to grow. Commonly available antimicrobial materials include Habasit AG’s HabaGUARD®, Sciessent’s Agion™, and antimicrobial technologies by Microban International, all of which use either silver zeolite or triclosan as antimicrobial agents. In these and many other antimicrobial materials, the antimicrobial agent is blended throughout the bulk of the material. The mechanism of activity is therefore by migration of the antimicrobial from the material to the food environment. The migratory nature of this mode of activity means that, eventually, the amount of antimicrobial agent capable of migrating to the food environment will diminish. As such, these materials are expected to exhibit high initial activity followed by a decline in activity, limiting long-term functionality. On the other hand, migratory antimicrobial materials have the capacity to exert activity against microorganisms that are not in direct contact with the material.
In contrast, antimicrobial agents that covalently adhere to a food processing surface are attached by the strongest possible chemical bond and are therefore less likely to migrate from the surface. Such non-migratory antimicrobial materials therefore have the potential for long-lasting antimicrobial activity and have the added benefit of being unlikely to migrate to the food product.
Unfortunately, most antimicrobial agents currently used in antimicrobial materials development lose activity after covalent attachment onto surfaces. Further, non-migratory antimicrobial materials require direct contact with microorganisms to be effective. Therefore, new approaches are needed in the design of antimicrobial materials in order to reap the benefits of high antimicrobial activity, long-lasting functionality, and suitability for the food processing environment. 
Nanotechnology-Enabled Interventions
Nanotechnology enables novel approaches toward the design of antimicrobial materials by the ability to manipulate matter and material chemistry at the nanoscale level. Several excellent reviews have been published that overview recent advances in nanotechnology and antimicrobial materials (Moraru et al., 2003; Moretro and Langsrud, 2011; Neethirajan and Jayas, 2011). Many approaches to using nanotechnology look at manipulating individual particles on the nanoscale; however, it may be possible to impart massive changes across a larger surface area by making nanoscale surface modifications.
Although food processing surfaces can be quite thick (on the order of millimeters to centimeters) and can extend over several square meters, the portion of that surface that interacts with the biological environment (e.g., food, microorganisms) is just the top several nanometers. Interestingly, by manipulating the chemistry and antimicrobial activity of just the top several nanometers, one can greatly influence how the material interacts with food components, microorganisms, and so on. Such nanosurface modification enables the retention of desirable bulk material properties (mechanical strength, thermal properties, machinability, etc.), which is an important consideration in commercial application of food processing surfaces.
--- PAGE BREAK ---
In nanosurface modification, the surface of a food contact or non-food contact material is modified to have desirable (e.g., antimicrobial) character. To be effective, however, the antimicrobial agent must retain activity after immobilization onto the material surface, a challenge for traditional antimicrobials. A new approach is in the development of surfaces that possess unique nanoscale chemical functionalities (i.e., N-halamines) capable of regenerating antimicrobial activity each time they are washed with a commonly used, food-approved halogen sanitizer.
Rechargeable Antimicrobial Materials
The nanoscale chemical functionalities that are capable of being repeatedly recharged with antimicrobial activity are called N-halamines. Halogenation of amides, amines, and imides results in the formation of an antimicrobial N-halamine structure (Akdag et al., 2006; Corral and Orazi, 1963; Goddard and Hotchkiss, 2008). Preliminary work suggests that the antimicrobial mechanism of N-halamine structure is by direct oxidation of essential biomolecules within a microorganism (DNA, RNA, lipids, proteins) and that the halogenated N-halamine structure is stable against dissociation into water. When put into contact with food pathogens or other microorganisms, the N-halamine structure exerts antimicrobial activity and reverts to its native form. These amides, amines, and imides can then be recharged with commercial halogen sanitizers (e.g., sodium hypochlorite) to regain their antimicrobial halogenated structure. This cycle can be repeated a number of times.
Commercial halogen-based chemical sanitizers (such as bleach, iodophors, and bromophors) are frequently used in food processing plants (Marriott, 1999) and are capable of generating the antimicrobial N-halamine structure. The major limitation to N-halamine antimicrobial materials is that, as with chlorine-based sanitizers, their activity is diminished with increasing organic load. Nevertheless, preliminary work has shown that N-halamine-modified materials can inhibit growth of E. coli K12 in the presence of organic matter (Goddard and Hotchkiss, 2008). Further, the widespread use of chlorine-based sanitizers, despite their known reduced effectiveness in the presence of high organic loads, suggests that the technology hurdle to overcome for adoption of N-halamine type antimicrobial materials is surmountable.
N-halamines were first investigated for use in antimicrobial textiles and non-woven fabrics to use in hospital linens to prevent nosocomial infections and for use in polystyrene beads and coatings suitable for pools and water disinfection applications (Sun and Sun, 2001; Chen et al., 2004; Sun and Sun, 2004; Tan and Obendorf, 2007; Worley and Williams, 1988). HaloSource®, Inc., markets water purification and textile coatings that leverage the N-halamine technology, but applications in food processing are limited. Such a strategy would be particularly suitable for use in a food processing environment, in which halogen-based sanitizers are commonly used during routine sanitation. During the subsequent processing run, the chlorinated N-halamine would inactivate microorganisms, providing a surface that resists colonization, multiplication, and cross-contamination of food pathogens and food spoilage organisms.
The session “Nanotechnology-Enabled Food Safety Interventions” at the 2011 IFT annual meeting demonstrated that model food contact surfaces had been modified to possess the antimicrobial N-halamines and subsequently exhibited antimicrobial activity against a range of spoilage and pathogenic organisms. Chlorine bleach (sodium hypochlorite) was used to generate the antimicrobial N-halamine structure; theoretically, iodophors and bromophors would work just as well.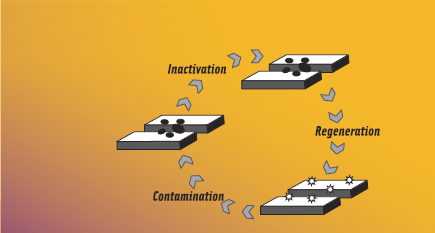
--- PAGE BREAK ---
In this research study, polyethylene substrates were modified by wet chemical or ultraviolet light oxidation, followed by immobilization of the N-halamine antmicrobial moieties. Control and modified substrates were subjected to repeated exposure to suspensions of Listeria monocytogenes, E. coli K12, Bacillus cereus, and Pseudomonas fluorescence. Materials modified by N-halamine were able to recharge their antimicrobial activity for each of the six successive tests and achieved at least 4-log reduction for all organisms tested.
Further tests with E. coli K12 showed the ability to achieve repeat antimicrobial activity after each of the four tests, in which materials were subjected to an antimicrobial activity assay, washed in an alkaline cleanser, recharged with chlorine, rinsed, and again tested for antimicrobial activity. Although these results are preliminary and there are notable limitations to the technology, the results indicate the potential for success in adopting N-halamine-based antimicrobial material technologies in some food applications.
Practical and Responsible Adaptation
Recent advances in nanotechnology, including N-halamine-modified materials, offer great promise towards improving the safety and sanitation of food contact and non-food contact materials. However, challenges remain in translating fundamental research into commercially practical solutions, and it is important to consider several factors. Of highest importance are cost, environmental impact, regulatory acceptance, material durability, and long-lasting antimicrobial activity.
Unfortunately, material durability and long-lasting antimicrobial activity are not regularly assessed in published literature, and standardized methodologies do not always accurately simulate the true wear and tear of a food processing environment. Specifically, it is important that materials be durable against repeat mechanical abrasion and exposure to chemical cleaners and sanitizers. For cases in which coatings may be considered, exposure to temperature extremes or other factors that might cause delamination should be rigorously assessed.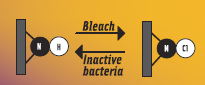
When interpreting and comparing the effectiveness of antimicrobial materials, it is often difficult to compare apples to apples. It is common to hear about commercial products that are able to kill 99% of disease-causing organisms, but the conditions that achieve the kill are often omitted. For example, how were the organisms enumerated? What medium was used? Was the microbial suspension sprayed on the material or was the material submerged in a microbial suspension? Further, particularly in the case of antimicrobial materials, microscopic evaluation of the material after enumeration is important to ensure that a perceived reduction in viable organisms is not simply a result of the organisms attaching onto the surface of the material. Providing that level of experimental detail is very important to accurately assess the practical applicability of individual antimicrobial material technologies.
--- PAGE BREAK ---
Finally, replacing standard food contact or non-food contact materials with antimicrobial versions is by no means a panacea or a replacement for needing to follow proper good manufacturing practices. Proper training and oversight at the implementation level are necessary to prevent the dangerous mindset of “why clean it if it’s antimicrobial?” Nevertheless, responsible development and use of antimicrobial materials used in concert with proper cleaning and sanitization protocols may help to reduce contamination by pathogenic or spoilage organisms.
Julie M. Goddard, a Professional Member of IFT, is Assistant Professor, Dept. of Food Science, University of Massachusetts, 102 Holdsworth Way, Amherst, Mass. 01003 ([email protected]).
This article is based in part on a paper presented during the session “Nanotechnology for Food Safety Intervention” at the IFT Annual Meeting & Food Expo, which took place June 2011 in New Orleans, La.
References
Akdag, A., Okur, S., McKee, M.L., Worley, S.D. 2006. The stabilities of N-Cl bonds in biocidal materials. J. Chem. Theory Comput. 2(3): 879–884.
Chen. Y., Worley, S.D., Huang, T.S., Weese, J., Kim, J., Wei, C.I., Williams, J.F. 2004. Biocidal polystyrene beads. Part III. Comparison of N-halamine and quat functional groups. J. Appl. Polym. Sci. 92(1): 363–367.
Corral, R.A. and Orazi, O.O. 1963. Substitution in hydantoin ring. Part 3. Halogenation. J. Org. Chem. 28(4):1100.
Goddard, J.M. and Hotchkiss, J.H. 2008. Rechargeable antimicrobial surface modification of polyethylene. J. Food Prot. 71(10): 2042–2047.
Moraru, C., Panchapakesan, C., Huang, Q., Takhistov, P., Liu, S., Kokini, J. 2003. Nanotechnology: a new frontier in food science. Food Technol. 57(12): 24–29.
Moretro, T. and Langsrud, S. 2011. Effects of materials containing antimicrobial compounds on food hygiene. J. Food Prot. 74(7): 1200–1211.
Neethirajan, S. and Jayas. D.S. 2011. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Tech. 4(1): 39–47.
Reij, M. and Den Aantrekker, E.D. 2004. Recontamination as a source of pathogens in processed foods. Int. J. Food Microbiol. 91(1): 1–11.
Scharff, R.L. 2010. Health-related costs from foodborne illness in the United States. Washington, DC: The Produce Safety Project at Georgetown University. p. 1–28.
Sun, Y.Y. and Sun, G. 2004. Novel refreshable N-halamine polymeric biocides: N-chlorination of aromatic polyamides. Ind. Eng. Chem. Rsch. 43(17): 5015–5020.
Tan, K.T. and Obendorf, S.K. 2007. Development of an antimicrobial microporous polyurethane membrane. J. Membrane Sci. 289(1-2): 199–209.
Todd, E.C.D., Greig, J.D., Michaels, B.S., Bartleson, C.A., Smith, D., Holah, J. 2010. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 11. Use of antiseptics and sanitizers in community settings and issues of hand hygiene compliance in health care and food industries. J. Food Prot. 73(12): 2306–2320.
Worley, S.D. and Williams, D.E. 1988. Halamine water disinfectants. Crit. Rev. Env. Contr. 18(2): 133–175.
